viết chuỗi phản ứng sau
H2SO4\(\rightarrow\)HCl\(\rightarrow\)SO2\(\rightarrow\)S03
Cân bằng các phản ứng sau theo phương pháp electron:
1. SO2 + H2S \(\rightarrow\) S + H2O
2. Al + Fe3O4 \(\rightarrow\) Al2O3 + Fe
3. SO2 + Cl2 + H2O \(\rightarrow\) H2SO4 + HCl
4. MnO2 + HCl \(\rightarrow\) MnCl2 + Cl2 + H2O
5. Cu + HNO3 \(\rightarrow\) Cu(NO3)2 + NO2 + H2O
6. Mg + H2SO4(n) \(\rightarrow\) MgSO4 + S + H2O
7*. FeSO4 + KMnO4 + H2SO4 \(\rightarrow\) Fe2(SO4)3 + MnSO4 + K2SO4 + H2O
8*. H2S + KMnO4 + H2SO4 \(\rightarrow\) MnSO4 + K2SO4 + S\(\downarrow\) + H2O
Hoàn thành chuỗi phản ứng sau.
\(C\rightarrow CO_2\rightarrow CaCO_3\rightarrow CaCl_2\rightarrow AgCl\)
\(C+O_2\underrightarrow{t^o}CO_2\)
\(CO_2+CaO\rightarrow CaCO_3\)
\(CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\)
\(CaCl_2+2AgNO_3\rightarrow Ca\left(NO_3\right)_2+2AgCl_{\downarrow}\)
Bạn tham khảo nhé!
\(C+O_2\underrightarrow{t^0}CO_2\)
\(CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3+H_2O\)
\(CaCO_3+2HCl\rightarrow H_2O+CO_2+CaCl_2\)
\(CaCl_2+2AgNO_3\rightarrow2AgCl+Ca\left(NO_3\right)_2\)
Chọn hệ số thích hợp để cân bằng các chất phản ứng sau:
1) Al + H2SO4 \(\rightarrow\) Al2(SO4)3 + H2
2) FeO + HCl \(\rightarrow\) FeCl2 + H2O
3) Na2O + H2O \(\rightarrow\) NaOH
4) N2O5 \(\rightarrow\) H2O \(\rightarrow\) HNO3
(Guys, please....help me)
3: \(Na_2O+H_2O\rightarrow2NaOH\)
1) 2Al + 3H2SO4 -> Al2(SO4)3 +3H2
2) FeO + 2HCl -> FeCl2 +H2O
3) Na2O + H2O -> 2NaOH
4) N2O5 + H2O -> 2HNO3
Hoàn thành chuỗi phản ứng trcacs phương trình
1 Mno\(_2\) \(\rightarrow Cl_2\rightarrow HCl\rightarrow NaCl\rightarrow Cl_2\rightarrow H_2SO_4\rightarrow HCl\)
2. \(KMnO_4\rightarrow Cl_2\rightarrow FeCl_3\rightarrow KCl\rightarrow KOH\)
3. \(HCl\rightarrow Cl_2\rightarrow FeCl_3\rightarrow NaCl\rightarrow HCl\rightarrow CuCl_2\rightarrow AgCl\)
4 \(NaCl\rightarrow HCl\rightarrow Cl_2\rightarrow KClO_3\rightarrow KCl\rightarrow Cl_2\rightarrow CaOCl_2\)
1/
MnO2+ 4HCl\(\rightarrow\) MnCl2+ Cl2+ 2H2O
Cl2+H2\(\xrightarrow[]{as}\) 2HCl
HCl+ NaOH\(\rightarrow\) NaCl+ H2O
2NaCl\(\xrightarrow[]{đpnc}\) 2Na+ Cl2
Cl2+ SO2+ 2H2O\(\rightarrow\) H2SO4+ 2HCl
H2SO4 đặc+ NaCltinh thể\(\xrightarrow[]{< 250\cdot C}\) NaHSO4+ HCl
4/
H2SO4 đặc+ NaCltinh thể\(\xrightarrow[]{< 250\cdot C}\) NaHSO4+ HCl
MnO2+ 4HCl\(\rightarrow\) MnCl2+ Cl2+ 2H2O
3Cl2+ 6KOHđặc\(\rightarrow\) KClO3+ 5KCl+ 3H2O
2KClO3\(\xrightarrow[MnO2]{to}\) 2KCl+ 3O2
2KCl\(\xrightarrow[]{đpnc}\) K+ Cl2
Cl2+ Ca(OH)2\(\xrightarrow[]{30\cdot C}\) CaOCl2+ H2O
1, \(MnO_2+4HCl\underrightarrow{t^o}MnCl_2+Cl_2+2H_2O\)
\(Cl_2+H_2\underrightarrow{as,t^o}2HCl\)
\(HCl+NaOH\rightarrow NaCl+H_2O\)
\(2NaCl+2H_2O\xrightarrow[cmn]{đpdd}2NaOH+Cl_2+H_2\)
\(4Cl_2+4H_2O+H_2S\rightarrow H_2SO_4+8HCl\)
\(H_2SO_4+NaCl\underrightarrow{>400^oC}Na_2SO_4+HCl\uparrow\)
2, \(2KMnO_4+18HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(3Cl_2+2Fe\underrightarrow{t^o}2FeCl_3\)
\(FeCl_3+3KOH\rightarrow Fe\left(OH\right)_3\downarrow+3KCl\)
\(2KCl+2H_2O\xrightarrow[cmn]{đpdd}2KOH+Cl_2+H_2\)
3, \(4HCl+MnO_2\underrightarrow{t^o}MnCl_2+Cl_2+2H_2O\)
\(3Cl_2+2Fe\underrightarrow{t^o}2FeCl_3\)\(FeCl_3+3NaOH\rightarrow3NaCl+Fe\left(OH\right)_3\downarrow\)
\(2NaCl+H_2SO_4\underrightarrow{>400^oC}Na_2SO_4+2HCl\)
\(2HCl+Cu\left(OH\right)_2\rightarrow CuCl_2+2H_2O\)
\(CuCl_2+2AgNO_3\rightarrow Cu\left(NO_3\right)_2+2AgCl\downarrow\)
4, \(H_2SO_4+2NaCl\underrightarrow{>400^oC}Na_2SO_4+2HCl\uparrow\)
\(4HCl+MnO_2\underrightarrow{t^o}MnCl_2+Cl_2+2H_2O\)
\(3Cl_2+6KOH\underrightarrow{80-100^oC}5KCl+KClO_3+3H_2O\)
\(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
\(2KCl+2H_2O\xrightarrow[cmn]{đpdd}2KOH+Cl_2+H_2\)
\(Cl_2+Ca\left(OH\right)_2\underrightarrow{30^oC}CaOCl_2+H_2O\)
Bạn tham khảo nhé!
1.
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+H_2O\)
\(Cl_2+H_2\underrightarrow{^{to}}2HCl\)
\(NaOH+HCl\rightarrow NaCl+H_2O\)
\(2NaCl\underrightarrow{^{đpnc}}2Na+Cl_2\)
\(Cl_2+SO_2+2H_2O\rightarrow2HCl+H_2SO_4\)
\(NaCl+H_2SO_{4_đ}\underrightarrow{^{to}}NaHSO_4+HCl\)
2.
\(2KMnO_4+16HCl\rightarrow2KCl+2MnCl_2+5Cl_2+8H_2O\)
\(2Fe+3Cl_2\underrightarrow{^{to}}2FeCl_3\)
\(FeCl_3+3KOH\rightarrow Fe\left(OH\right)_3+3KCl\)
\(2KCl+2H_2O\underrightarrow{^{đpmn}}2KOH+H_2+Cl_2\)
3.
\(MnO_2+4HCl\underrightarrow{^{to}}MnCl_2+Cl_2+2H_2O\)
\(2Fe+3Cl_2\underrightarrow{^{to}}2FeCl_3\)
\(FeCl_3+3NaOH\rightarrow Fe\left(OH\right)_3+3NaCl\)
\(NaCl+H_2SO_{4_Đ}\underrightarrow{^{to}}NaHSO_4+HCl\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(CuCl_2+2AgNO_3\rightarrow2AgCl+Cu\left(NO_3\right)_2\)
4.
\(NaCl+H_2SO_{4_Đ}\underrightarrow{^{to}}NaHSO_4+HCl\)
\(MnO_2+4HCl\rightarrow MnCl_2+Cl_2+2H_2O\)
\(3Cl_2+6KOH\underrightarrow{^{to}}5KCl+KClO_3+3H_2O\)
\(KClO_3+6HCl\rightarrow KCl+3Cl_2+3H_2O\)
\(Cl_2+CaO\underrightarrow{^{to}}CaOCl_2\)
Hoàn thành các PT phản ứng thực hiện chuỗi biến hóa sau:
a) \(KClO_3\rightarrow X\rightarrow Y\rightarrow Z\rightarrow T\rightarrow Al_2\left(SO_4\right)_3\)
b) \(KMnO_4\rightarrow A\rightarrow H_2O\rightarrow B\rightarrow H_2\rightarrow C\)
a)
\(2KClO_3 \xrightarrow{t^o} 2KCl + 3O_2(X)\\ 3O_2 + 4Al \xrightarrow{t^o} 2Al_2O_3(Y)\\ Al_2O_3 + 6HCl \to 2AlCl_3(Z) + 3H_2O\\ AlCl_3 + 3KOH \to Al(OH)_3(T) + 3KCl\\ 2Al(OH)_3 + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2O\\ \)
b)
\(2KMnO_4 \xrightarrow{t^o} K_2MnO_4 + MnO_2 + O_2(A)\\ 2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ 2H_2O + 2Na \to 2NaOH(B) + H_2\\ 2NaOH \xrightarrow{đpnc} Na + O_2 + H_2 CuO + H_2 \xrightarrow{t^o} Cu(C) + H_2O\)
A: O2 B: Al2O3 C: AlCl3 D: Al(OH)3
2KClO3-to-->2KCl+3O2
3O2+4Al--->2Al2O3
Al2O3+6HCl--->2AlCl3+3H2O
AlCl3+3NaOH--->Al(OH)3+3NaCl
2Al(OH)3+3H2SO4--->Al2(SO4)3+6H2O
b> 2
Viết PTHH thể hiện chuỗi phản ứng sau
a)FeS2\(\rightarrow SO_2\rightarrow SO_3\rightarrow H_2SO_4\rightarrow CuSO_4\)
b)\(AlCl_3\rightarrow Al\left(OH\right)_3\rightarrow Al_2O_3\rightarrow Al\left(SO_4\right)_3\rightarrow AlCl_3\)
c)\(Na\rightarrow Na_2O\rightarrow NaOH\rightarrow Na_2CO_3\rightarrow NaHCO_3\)
a) 4FeS2+11O2\(\overset{t^0}{\rightarrow}\)2Fe2O3+8SO2
2SO2+O2\(\overset{V_2O_5,t^0}{\rightarrow}2SO_3\)
SO3+H2O\(\rightarrow\)H2SO4
Cu+ 2H2SO4đ\(\overset{t^0}{\rightarrow}\)CuSO4+SO2+H2O
b)
AlCl3+3NaOH\(\rightarrow\)Al(OH)3+3NaCl
2Al(OH)3\(\overset{t^0}{\rightarrow}Al_2O_3+3H_2O\)
Al2O3+3H2SO4\(\rightarrow\)Al2(SO4)3+3H2O
Al2(SO4)3+3BaCl2\(\rightarrow\)3BaSO4+2AlCl3
c) 4Na+O2\(\overset{t^0}{\rightarrow}2Na_2O\)
Na2O+H2O\(\rightarrow\)2NaOH
2NaOH+CO2\(\rightarrow\)Na2CO3+H2O
Na2CO3+CO2+H2O\(\rightarrow\)2NaHCO3
- Na2O + H2O --> 2NaOH
- SO2 + 2 NaOH --> Na2SO3+ H2O
- Na2SO3 + H2SO4 --> Na2SO4 + SO2 + H2O
- SO2 + K2O --> K2SO3
Thực hiện chuỗi phản ứng sau:
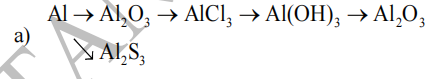
b) \(Al_2O_3\rightarrow Al\rightarrow Al_2\left(SO_4\right)_3\rightarrow AlCl_3\rightarrow Al\left(OH\right)_3\rightarrow Al_2O_3\)
c) \(Al\rightarrow AlCl_3\rightarrow Al\left(OH\right)_3\rightarrow Al_2O_3\rightarrow Al_2\left(SO_4\right)_3\rightarrow AlCl_3\)
a)\(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
\(2AlCl_3+3Ba\left(OH\right)_2\rightarrow2Al\left(OH\right)_3\downarrow+3BaCl_2\)
\(2Al\left(OH\right)_3\underrightarrow{t^o}Al_2O_3+3H_2O\)
\(2Al+3S\underrightarrow{t^o}Al_2S_3\)
Viết PTHH hoàn thành các chuỗi phản ứng sau
1, \(Al\rightarrow Al_2O_3\rightarrow AlCl_3\rightarrow Al\left(OH\right)_3\rightarrow Al_2O_3\rightarrow Al\rightarrow AlCl_3\)
2,\(Fe\rightarrow FeSO_4\rightarrow Fe\left(OH\right)_2\rightarrow FeCl_2\)
3,\(Al\rightarrow AlCl_3\rightarrow Al\left(OH\right)_3\rightarrow Al_2O_3\rightarrow Al_2\left(SO_4\right)_3\rightarrow AlCl_3\rightarrow MgCl_2\)
giúp mk với, mk cảm ơn
1, (1) 4Al+ 3O2--(to)---> 2Al2O3
(2) Al2O3+6HCl---> 2AlCl3+ 3H2O
(3) AlCl3+3NaOH---->Al(OH)3+ 3NaCl
(4) 2Al(OH)3---(to)---> Al2O3+ 3H2O
(5)2Al2O3---(to+ đpnc)--> 4Al+ 3O2
(6) 2Al+ 3Cl2---> 2AlCl3
2, (1)Fe+ CuSO4-->Cu+ FeSO4
(2) FeSO4+ 2NaOH--> Fe(OH)2+ Na2SO4
(3) Fe(OH)2 +2HCl---> FeCl2+ 2H2O
Bài cuối tự làm nhé!
câu 3
2Al + 3Cl2 --t--> 2AlCl3
AlCl3 + 3NaOH -> 3NaCl + Al(OH)3
2Al(OH)3 -t-> Al2O3 + H2O
Al2O3 + 3H2SO4 -> Al2(SO4)3 + 3H2O
Al2(SO4)3 + 3BaCl2 -> 3BaSO4 + 2AlCl3
2AlCl3 + 3Mg -> 2Al + 3MgCl2
\(\left(1\right)4Al+3O_2\underrightarrow{t^o}2Al_2O_3\\ \left(2\right)Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\\ AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3\downarrow+3NaCl\\ 2Al\left(OH\right)_3\underrightarrow{t^o}Al_2O_3+3H_2O\\ 2Al_2O_3\underrightarrow{dpnc}4Al+3O_2\\ 2Al+6HCl\rightarrow2AlCl_3+3H_2\)