Giải giúp em bài này với ạ em cảm ơn 

Những câu hỏi liên quan
Mọi người giải giúp em bài này với ạ sẵn giải thích giùm em luôn ạ . Giải nhanh giúp em. Em cảm ơn. 
III.
A.
1. B
2. C
3. B
4. A
5. D
B.
1. C
2. B
3. B
4. A
5. C
Đúng 2
Bình luận (0)
1 b c b a d
2 c b b a c nha
Mọi người giải giúp em bài này với ạ . Giải nhanh giúp em. Em cảm ơn. 
1 The meeting was canceled 3 days ago
2 She told me she was watching a film with her sister then
3 I admire the guitarist who is perfroming on the stage
4 Had it not been for Pauline's interest, the project would have been abandoned
Đúng 2
Bình luận (0)
IV.
1. The meeting was canceled 3 days ago.
2. She told me shewas watching a film with her sister then
3. I admire the guitarist who is performing on the stage
4. Had it not been for Pauline's interest, the project was abandoned. (chắc thế)
Đúng 0
Bình luận (0)
Giải giúp em bài này với ạ,em cảm ơn !!
He asked me to know what the time was.
She asked me when we would meet again.
She asked him If he was crazy.
He asked me to know when they had lived.
He asked her If she would be at the party.
She asked me If I could meet her at the station.
The teacher asked me to know who knew the answer
She asked him to know why he didn't help her.
He asked me If I had seen that car.
The mother asked the twins If they had tidied up their room.
Tom asked how much that computer was
Đúng 1
Bình luận (1)
11. what the time was.
12. when they would meet again.
13. if he was crazy.
14. where they had lived.
15. if she would be at the party.
16. if I could meet her at the station.
17. who knew the answer.
18. why I didn't help her.
19. if I had seen that car.
20. if they had tidied up their room.
Bài 3:
asked how much that computer was.
Đúng 1
Bình luận (1)
Giải giúp em bài này với ạ. Em cảm ơn
Ta có: \(\left\{{}\begin{matrix}p+e+n=52\\p=e\\n-p=1\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}p=e=17\\n=18\end{matrix}\right.\)
Đúng 1
Bình luận (0)
Giúp em giải bài này với ạ em cảm ơn
Đọc tiếp
Giúp em giải bài này với ạ em cảm ơn
Giúp em giải bài này với ạ! Em cảm ơn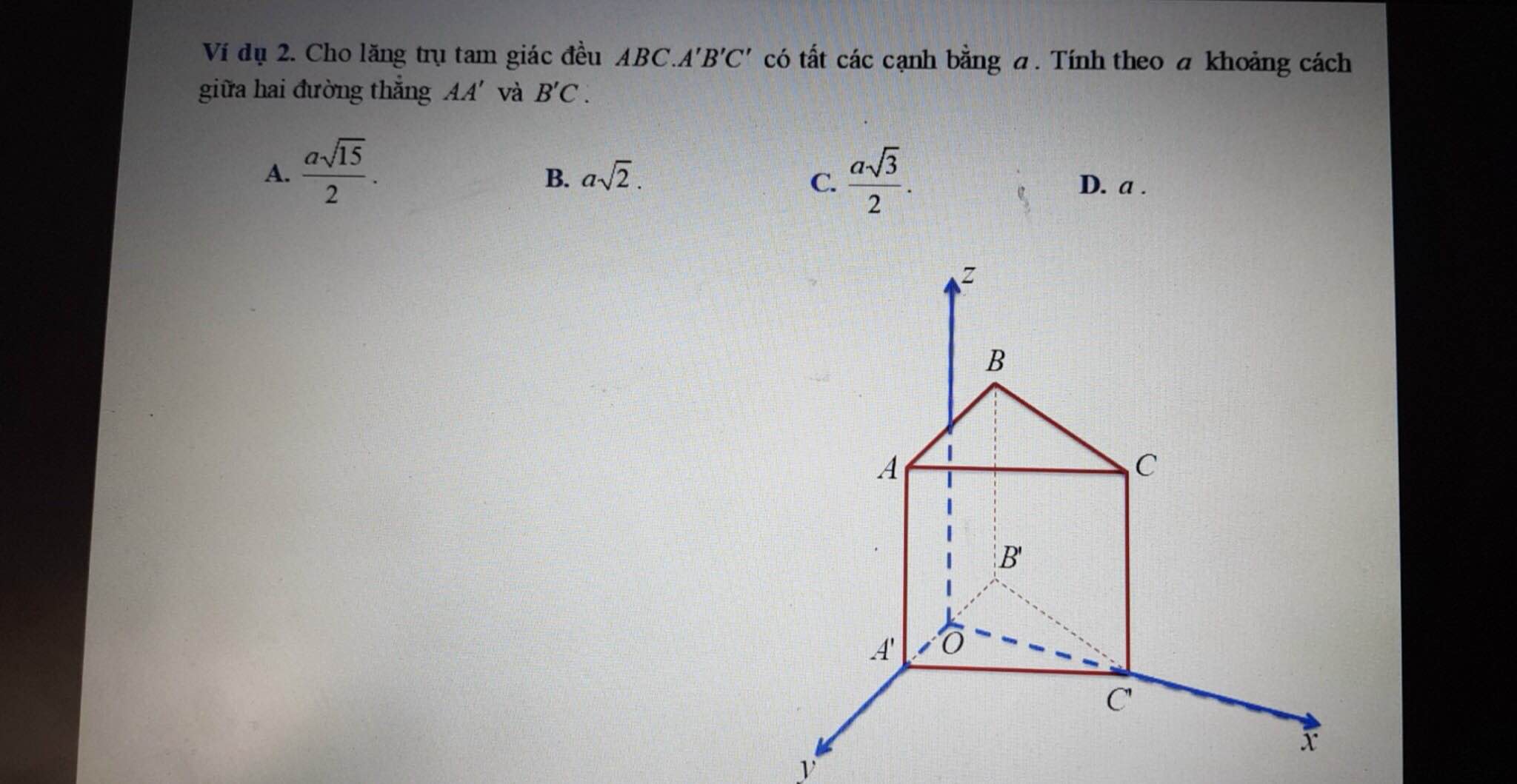
Giải giúp em bài này với ạ em cảm ơn 
Câu 2 :
a) PTHH : \(2Al+3S-->Al_2S_3\)
\(Zn+S-->ZnS\)
mS = 29,55 - 15,15 = 14,4 (g)
b) nS = 14,4/32 = 0,45 (mol)
PTHH : \(Al_2S_3+3H_2SO_4-->Al_2\left(SO_4\right)_3+3H_2S\)
\(ZnS+H_2SO_4-->ZnSO_4+H_2S\)
\(H_2S+2AgNO_3-->Ag_2S+2HNO_3\)
Bảo toàn S : nAg2S = nH2S = nS = 0,45 (mol)
=> mktAg2S = 111,6 (g)
c) PTHH : \(H_2S+CuCl_2-->CuS+2HCl\)
nH2S = 0,45/2 = 0,225 (mol)
Theo pthh : nCuS = nH2S = 0,225 (mol)
=> mCuS = 0,225.96 = 21,6 (g)
Đúng 0
Bình luận (0)
Giúp em giải bài này với ạ! Em cảm ơn 
a)
Gọi số mol Fe, Al là a, b (mol)
=> 56a + 27b = 8,94 (1)
\(n_{H_2}=\dfrac{0,36}{2}=0,18\left(mol\right)\)
PTHH: Fe + 2HCl --> FeCl2 + H2
a------------->a----->a
2Al + 6HCl --> 2AlCl3 + 3H2
b---------------->b------>1,5b
=> a + 1,5b = 0,18 (2)
(1)(2) => a = 0,15 (mol); b = 0,02 (mol)
=> \(\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,15.56}{8,94}.100\%=93,96\%\\\%m_{Al}=\dfrac{0,02.27}{8,94}.100\%=6,04\%\end{matrix}\right.\)
b)
mFeCl2 = 0,15.127 = 19,05 (g)
mAlCl3 = 0,02.133,5 = 2,67 (g)
Đúng 4
Bình luận (0)
Giúp em giải bài này với ạ! Em cảm ơn 
Gọi số mol Mg, Zn là a, b (mol)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: Mg + 2HCl --> MgCl2 + H2
a---------------->a----->a
Zn + 2HCl --> ZnCl2 + H2
b--------------->b---->b
=> a + b = 0,3 (1)
Và 95a + 136b = 36,7 (2)
(1)(2) => a = 0,1 (mol); b = 0,2 (mol)
\(\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,1.24}{0,1.24+0,2.65}.100\%=15,6\%\\\%m_{Zn}=\dfrac{0,2.65}{0,1.24+0,2.65}.100\%=84,4\%\end{matrix}\right.\)
Đúng 3
Bình luận (0)
Giúp em giải bài này với ạ
Em cảm ơn 
a, Hiện tượng: mất dần màu vàng lục của Clo, có khí không màu thoát ra
\(K_2CO_3+Cl_2\rightarrow KCl+KClO+CO_2\uparrow\)
b, \(F_2+H_2\xrightarrow[\text{nhiệt độ âm}]{\text{bóng tối}}2HF\)
\(Cl_2+H_2\underrightarrow{as}2HCl\)
\(Br_2+H_2\underrightarrow{t^o}2HBr\\ I_2+H_2\underrightarrow{t^o,xt}2HI\)
Đúng 1
Bình luận (0)




















