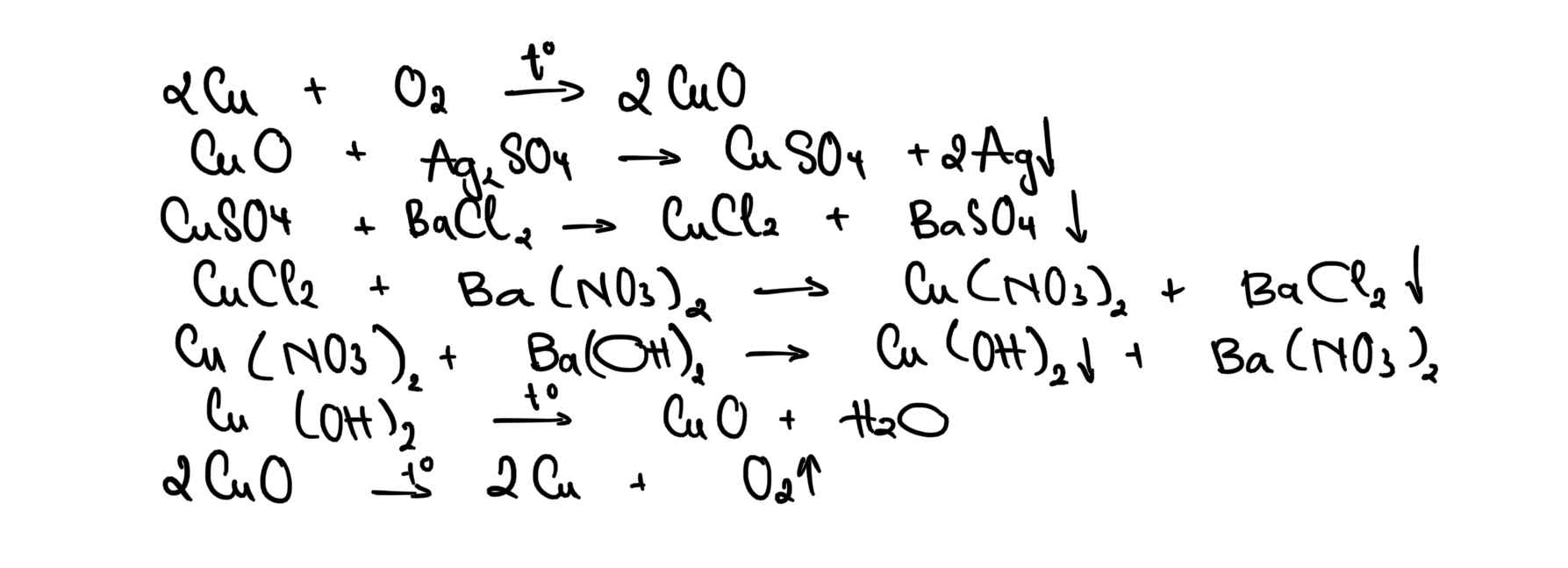CuSO4 -> CuCl2 -> CuCO -> CuO -> Cu(NO3)2

Những câu hỏi liên quan
Cu -> CuO -> CuSO4 -> CuCl2 -> Cu(NO3)2 -> Cu(OH)2 -> CuO -> Cu
\(Cu\overset{\left(1\right)}{-->}CuO\overset{\left(2\right)}{-->}CuSO_4\overset{\left(3\right)}{-->}CuCl_2\overset{\left(4\right)}{-->}Cu\left(NO_3\right)_2\overset{\left(5\right)}{-->}Cu\left(OH\right)_2\overset{\left(6\right)}{-->}CuO\overset{\left(7\right)}{-->}Cu\)
\(\left(1\right)2Cu+O_2\overset{t^o}{--->}2CuO\)
\(\left(2\right)CuO+H_2SO_4--->CuSO_4+H_2O\)
\(\left(3\right)CuSO_4+BaCl_2--->BaSO_4\downarrow+CuCl_2\)
\(\left(4\right)CuCl_2+2AgNO_3--->2AgCl\downarrow+Cu\left(NO_3\right)_2\)
\(\left(5\right)Cu\left(NO_3\right)_2+2NaOH--->Cu\left(OH\right)_2\downarrow+2NaNO_3\)
\(\left(6\right)Cu\left(OH\right)_2\overset{t^o}{--->}CuO+H_2O\)
\(\left(7\right)CuO+H_2\overset{t^o}{--->}Cu+H_2O\)
Đúng 2
Bình luận (0)
\(2Cu+O_2\underrightarrow{t^o}2Cuo\)
\(CuO+H_2SO_4->CuSO_4+H_2O\)
\(CuSO_4+BaCl_2->BaSO_4\downarrow+CuCl_2\)
\(CuCl_2+2AgNO_3->Cu\left(NO_3\right)_2+2AgCl\downarrow\)
\(Cu\left(NO_3\right)_2+2NaOH->Cu\left(OH\right)_2\downarrow+2NaNO_3\)
\(Cu\left(OH\right)_2\underrightarrow{t^o}CuO+H_2O\)
\(CuO+H_2\underrightarrow{t^o}Cu+H_2O\)
Đúng 1
Bình luận (0)
CuO,CuSO4,Cu(OH)2,CuCl2,Cu(NO3)2,Cu
Tham khảo
(1) 2Cu + O2 ---> 2CuO
(2) CuO + H2SO4 ---> CuSO4 + H2
(3) CuSO4 + 2NaOH ---> Cu(OH)2 + Na2SO4
(4) Cu(OH)2 + 2HCl ---> CuCl2 + 2H2O
(5) CuCl2 + 2AgNO3 ----> Cu(NO3)2 + 2AgCl
Đúng 1
Bình luận (0)
Cu->CuO->CuCl2->Cu(OH)2->CuSO4->Cu(NO3)2
help mik vs
\(2Cu+O_2\underrightarrow{t^o}2CuO\)
\(CuO+2HCl->CuCl_2+H_2O\)
\(CuCl_2+2NaOH->Cu\left(OH\right)_2\downarrow+2NaCl\)
\(Cu\left(OH\right)_2+H_2SO_4->CuSO_4+2H_2O\)
\(CuSO_4+Ba\left(NO_3\right)_2->Cu\left(NO_3\right)_2+BaSO_4\downarrow\)
Đúng 2
Bình luận (1)
Viết phương trình Cu -> Cucl2 -> Cu(No3)2 -> Cu(OH)2 -> CuO -> CuSo4 -> MgSo4
\(Cu+Cl_2\xrightarrow[]{t^0}CuCl_2\\ CuCl_2+2AgNO_3\rightarrow Cu\left(NO_3\right)_2+2AgCl\\ Cu\left(NO_3\right)_2+2KOH\rightarrow Cu\left(OH\right)_2+2KNO_3\\ Cu\left(OH\right)_2\xrightarrow[]{t^0}CuO+H_2O\\ CuO+H_2SO_4\rightarrow CuSO_4+H_2O\\ CuSO_4+Mg\rightarrow MgSO_4+Cu\)
Đúng 2
Bình luận (0)
Cu+Cl2➝CuCl2
CuCl2+2AgNO3➝Cu(NO3)2+2AgCl↓
Cu(NO3)2+2NaOH➝ Cu(OH)2↓+2NaNO3
Cu(OH)2➝CuO+H2O
CuO+H2SO4➝CuSO4+H2O
CuSO4+Mg(OH)2➝Cu(OH)2↓+MgSO4
Đúng 1
Bình luận (0)
Hoàn thành sơ đồ phản ứng :
Cu-->CuO-->CuCl2-->Cu(OH)2-->CuSO4-->Cu(NO3)2
\(2Cu+O_2\underrightarrow{^{^{t^o}}}2CuO\)
\(CuO+2HCl\rightarrow CuCl_2+H_2O\)
\(CuCl_2+2NaOH\rightarrow Cu\left(OH\right)_2+2NaCl\)
\(Cu\left(OH\right)_2+H_2SO_4\rightarrow CuSO_4+2H_2O\)
\(CuSO_4+Ba\left(NO_3\right)_2\rightarrow Cu\left(NO_3\right)_2+BaSO_4\)
Đúng 4
Bình luận (0)
Cu --(Oxi hóa)--> CuO --(Phản ứng với HCl)--> CuCl2 --(Phản ứng với NaOH)--> Cu(OH)2 --(Phản ứng với H2SO4)--> CuSO4 --(Phản ứng với HNO3)--> Cu(NO3)2
____________________HT_________________________
Đúng 1
Bình luận (0)
Viết phương trình hóa học hoàn thành dãy chuyển hóa sau: Cu->CuO->Cucl2->Cu(OH)2->CuO->CuSO4->Cu(NO3)2
2Cu + O2 ---> 2CuO
CuO + HCl ---> CuCl2 + H2O
CuCl2 + Ba(OH)2 ---> Cu(OH)2 + BaCl2
Cu(OH)2 ---to--> CuO + H2O
CuO + SO3 ---> CuSO4
CuSO4 + Pb(NO3)2 --->Cu(NO3)2 + PbSO4
Đúng 1
Bình luận (0)
em tham khảo nha
https://hoctap247.com/de-thi-kiem-tra/cau-hoi/1531862-viet-cac-phuong-trinh-hoa-hoc-tuong-ung-voi-day-chuyen-hoa-sau-cu-1cucl2-2cuno32-3cuoh2-4cuo.html
Đúng 1
Bình luận (0)
Câu 4: Viết PTHH thực hiện chuỗi biến hóa sau:
Cu(OH)2 ->CuO -> CuSO4 ->CuCl2 ->Cu(NO3)2 -> Cu -> CuO.
$Cu(OH)_2 \xrightarrow{t^o} CuO + H_2O$
$CuO + H_2SO_4 \to CuSO_4 + H_2O$
$CuSO_4 + BaCl_2 \to BaSO_4 + CuCl_2$
$CuCl_2 + 2AgNO_3 \to 2AgCl + Cu(NO_3)_2$
$Cu(NO_3)_2 + Fe \to Cu + Fe(NO_3)_2$
$2Cu + O_2 \xrightarrow{t^o} 2CuO$
Đúng 2
Bình luận (0)
Viết PTHH cho những chuyển đổi hh. CuO---->CuSO4--->Cu(NO3)2--->Cu(OH)2---->CuO--->CuCl2--->Cu (mọi ng giúp e với ạ)
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\\ CuSO_4+Ba\left(NO_3\right)_2\rightarrow BaSO_4+Cu\left(NO_3\right)_2\\ Cu\left(NO_3\right)_2+2KOH\rightarrow Cu\left(OH\right)_2+2KNO_3\\ Cu\left(OH\right)_2\rightarrow\left(t^o\right)CuO+H_2O\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ CuCl_2\rightarrow\left(t^o\right)Cu+Cl_2\)
Đúng 4
Bình luận (0)
Viết phương trình thực hiện dãy chuyển hóa sau
CuO --> CuSO4 --> Cu(NO3)2 --> Cu(OH)2 --> CuO --> CuCl2 --> Cu