
Cứu e vs mn
 Cứu e vs mn ơi
Cứu e vs mn ơi
2. Can you remember to see my keys? I can't find them.
3. Oh no! I forgot to send my mom a birthday card! She'll be ready upset.
4. I would have arrived earlier, but I had to stop to get some gas for my car.
5. I try to do some exercise every day, but I often can't find the time.
6. You can forget to go out tonight! You haven't done your homework.
7. Please stop talking for a minute and just listen to me.
8. He's been trying to learn to drive for years but keeps failing the test.
9. I've stopped using my car so much and walked as much as I can.
10. You must remember to visit Aunt Anna when you're in town.
11. I was really hot, so I tried to splash my face with cold water.
12. Remember to turn off your phone before you enter.
13. I don't remember meeting her at your wedding at all.
14. She's not interested in you, so you can forget to ask her out.
15. I felt sick so I tried to drink some ginger tea.
16. My children never remember putting the milk back in the refrigerator.
17. I stopped drinking so much caffeine and now I feel much better.
18. We've been trying to get the car out all morning, but the snow is just too deep.
19. We'll have to stop to buy him a present on the way to the party.
20. I didn't remember sending that email before I left the office.
21. I think I forgot to turn off the iron before I left.
22. Why don't you stop playing on your computer and go outside for some fresh air?
23. Could you try to remember people's names, please? It's really rude to keep forgetting.
24. If he's not answering his telephone, you could try to get in touch with him by email.
phần con lại cj gửi nhé
1 taking
2 seeing
3 to send
4 to get
5 to do
6 to go
Mn cứu e bài sắp xếp đi
Đây ko phải bài thi nên mn giúp e vs
Nếu ai dùng thì bt Shub class. Edu chỉ là ứng dụng giao bt chứ ko phải để thi
Em xin trân trọng cảm ơn
cứu vs mn ;-;
1.She wants to as lose weight so as she is on a diet.
2.He got up early this morning so as he wouldn't be late for work.
Cứu vs mn oi
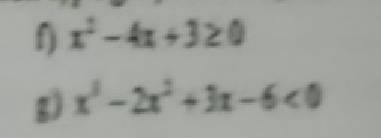
cứu vs mn ơi
Bạn ơi câu g mờ quá bạn gửi lại ảnh mình làm tiếp nhé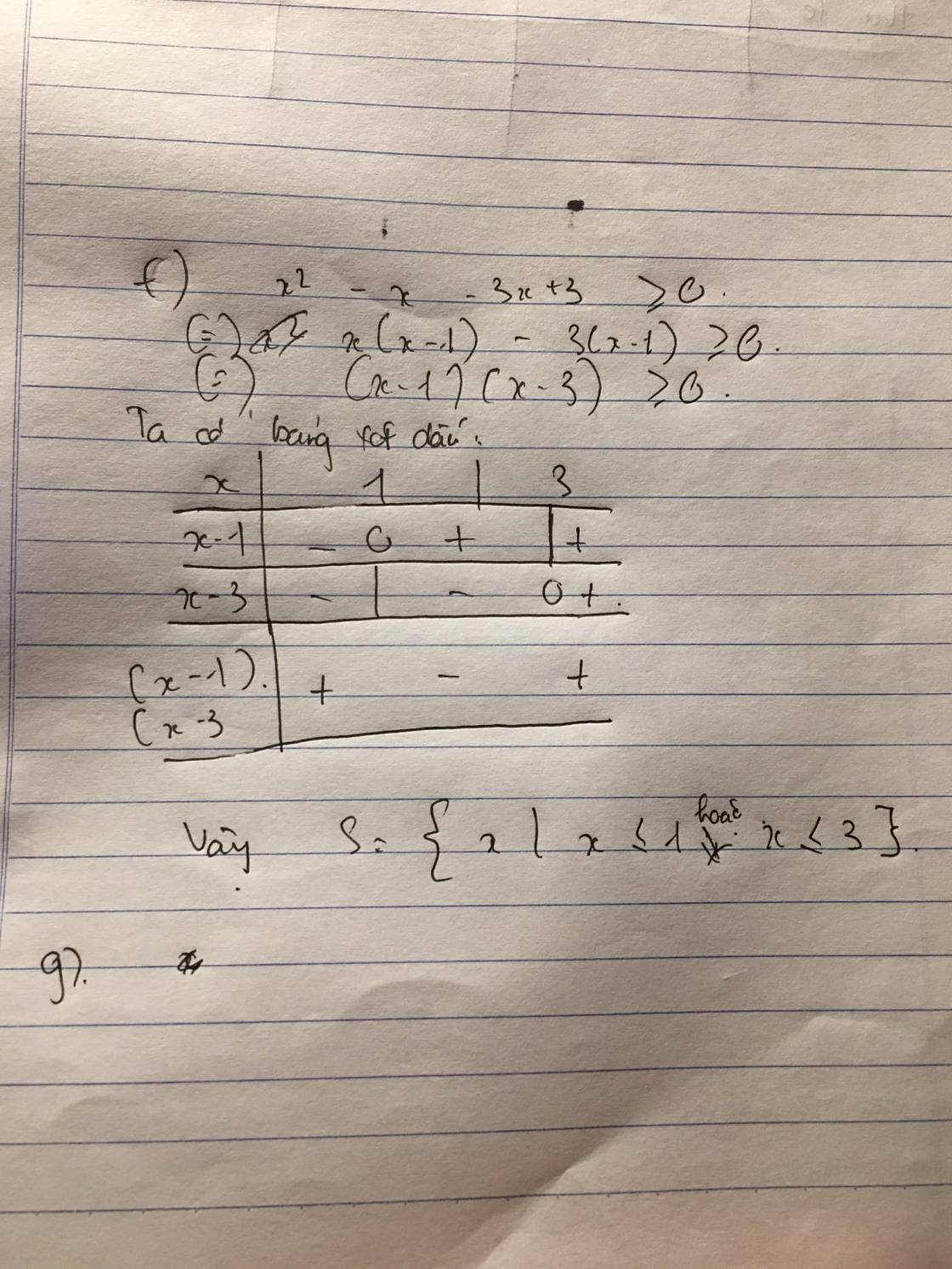
Cứu mik vs mn ơi
→ PTHH cân bằng:
\(2 N H_{3} + 3 C l_{2} \rightarrow N_{2} + 6 H C l\)
Chất khử: NH₃, chất oxi hóa: Cl₂
2. NH₃ + O₂ → NO + H₂O\(4 N H_{3} + 5 O_{2} \rightarrow 4 N O + 6 H_{2} O\)
Chất khử: NH₃ (N: -3 → +2)
Chất oxi hóa: O₂ (0 → -2)
\(8 A l + 3 F e_{3} O_{4} \rightarrow 4 A l_{2} O_{3} + 9 F e\)
Chất khử: Al (0 → +3)
Chất oxi hóa: Fe₃O₄ (Fe³⁺, Fe²⁺ → Fe⁰)
\(M n O_{2} + 4 H C l \rightarrow M n C l_{2} + C l_{2} + 2 H_{2} O\)
Chất khử: HCl (Cl⁻ → Cl₂)
Chất oxi hóa: MnO₂ (Mn⁴⁺ → Mn²⁺)
\(2 K M n O_{4} + 16 H C l \rightarrow 2 M n C l_{2} + 5 C l_{2} + 8 H_{2} O + 2 K C l\)
Chất khử: HCl
Chất oxi hóa: KMnO₄
\(3 C u + 8 H N O_{3} \rightarrow 3 C u \left(\right. N O_{3} \left.\right)_{2} + 2 N O + 4 H_{2} O\)
Chất khử: Cu
Chất oxi hóa: HNO₃ (N⁺⁵ → N⁺²)
\(4 Z n + 10 H N O_{3} \rightarrow 4 Z n \left(\right. N O_{3} \left.\right)_{2} + N H_{4} N O_{3} + 3 H_{2} O\)
8. Al + HNO₃ → Al(NO₃)₃ + N₂O + H₂O\(8 A l + 30 H N O_{3} \rightarrow 8 A l \left(\right. N O_{3} \left.\right)_{3} + 3 N_{2} O + 15 H_{2} O\)
9. Al + H₂SO₄ (đặc) → Al₂(SO₄)₃ + SO₂ + H₂O\(2 A l + 6 H_{2} S O_{4} \left(\right. đặ c \left.\right) \rightarrow A l_{2} \left(\right. S O_{4} \left.\right)_{3} + 3 S O_{2} + 6 H_{2} O\)
10. KMnO₄ + FeSO₄ + H₂SO₄ → MnSO₄ + Fe₂(SO₄)₃ + K₂SO₄ + H₂O\(2 K M n O_{4} + 10 F e S O_{4} + 8 H_{2} S O_{4} \rightarrow K_{2} S O_{4} + 2 M n S O_{4} + 5 F e_{2} \left(\right. S O_{4} \left.\right)_{3} + 8 H_{2} O\)
11. K₂Cr₂O₇ + FeSO₄ + H₂SO₄ → Cr₂(SO₄)₃ + Fe₂(SO₄)₃ + K₂SO₄ + H₂O\(K_{2} C r_{2} O_{7} + 6 F e S O_{4} + 7 H_{2} S O_{4} \rightarrow K_{2} S O_{4} + C r_{2} \left(\right. S O_{4} \left.\right)_{3} + 3 F e_{2} \left(\right. S O_{4} \left.\right)_{3} + 7 H_{2} O\)
12. H₂O₂ + KMnO₄ + H₂SO₄ → O₂ + MnSO₄ + K₂SO₄ + H₂O\(2 K M n O_{4} + 5 H_{2} O_{2} + 3 H_{2} S O_{4} \rightarrow K_{2} S O_{4} + 2 M n S O_{4} + 8 H_{2} O + 5 O_{2}\)
13. Cl₂ + KOH (t°) → KCl + KClO₃ + H₂O\(3 C l_{2} + 6 K O H \overset{t °}{\rightarrow} 5 K C l + K C l O_{3} + 3 H_{2} O\)
14. Al + HNO₃ → Al(NO₃)₃ + NO + N₂O + H₂O (V_NO : V_N2O = 3:1)→ Giải tỉ lệ khí:
\(8 A l + 37 H N O_{3} \rightarrow 8 A l \left(\right. N O_{3} \left.\right)_{3} + 3 N O + N_{2} O + 18 H_{2} O\)
15. FeS + H₂SO₄ (đặc, nóng) → Fe₂(SO₄)₃ + SO₂ + H₂O\(2 F e S + 8 H_{2} S O_{4} \rightarrow F e_{2} \left(\right. S O_{4} \left.\right)_{3} + 8 S O_{2} + 8 H_{2} O\)
 cứu mik vs mn ơiiiiiiiiiiiiii
cứu mik vs mn ơiiiiiiiiiiiiii
21C (information không đếm được, cụm collocation provide with)
22A (cụm collocation Listening to the radio, và hai sở thích của người viết trong câu trên là số nhiều)
23B (thì tương lai đơn)
24D (cụm collocation In the world, The most popular)
25 có lẽ phải xem lại
mn cứu e với
Cứu e với mn