Giúp mình vs ạ, mình sẽ fl ạ, mình đang gấp lắm

Những câu hỏi liên quan
Mình đang cần gấp ạ, mình sẽ fl ạ
Giúp mình vs ạ . Mình đang gấp lắm
3 careless
4 bleed
V
1 Hung has colllected stamps since 2000
2 The front yard isn't large enough to play soccer in
3 Milk is delivered twice a week by the milkman
4 When did you bought this computer?
5 His car doesn't run as fasst as a race car
6 Two languages can be spoken well by Lan
VI
1 T
2 F
3 T
4 T
VII
1 Mr. Nam is arriving in Hue tonight
2 They are going back to Englang in two months
3 It is not difficult to tránlate this sentence into English
4 Would you mind lending me your dictionary?
Đúng 1
Bình luận (0)
3 careless
4 bleed
V
1 Hung has colllected stamps since 2000
2 The front yard isn't large enough to play soccer in
3 Milk is delivered twice a week by the milkman
4 When did you bought this computer?
5 His car doesn't run as fasst as a race car
6 Two languages can be spoken well by Lan
VI
1 T
2 F
3 T
4 T
VII
1 Mr. Nam is arriving in Hue tonight
2 They are going back to Englang in two months
3 It is not difficult to tránlate this sentence into English
4 Would you mind lending me your dictionary?
Mình đang cần gấp, mình sẽ fl ạ
Mình đang cần gấp, mình sẽ fl ạ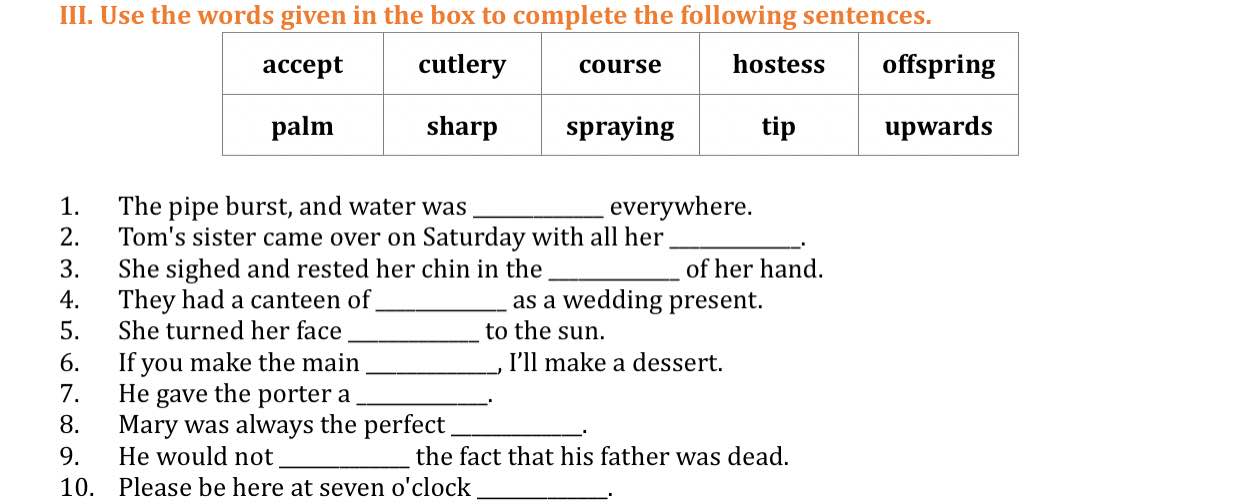
1. spraying
2. offspring
3. palm
4. cutlery
5. upwards
6. course
7. tip
8. hostess
9. accept
10. sharp
Đúng 1
Bình luận (0)
Mình đang gấp lắm mn ơi, nhờ mn giúp mình câu 3 vs ạ, nếu đc cả câu 4 nữa thì mình cảm ơn mn nhiều.

giúp mình với ạ, gấp lắm, mình đang ktra ạ
Làm nhanh hộ mình vs ạ . Mình đang gấp lắm
I
1C
2 D
3A
4 B
II
5 D
6 A
7 D
8 B
9 C
10 A
11 B
12 B
13 B
14 D
Đúng 3
Bình luận (0)
 Giúp mình với ạ mình đang cần gấp lắm :'(( mình cảm ơn ạ
Giúp mình với ạ mình đang cần gấp lắm :'(( mình cảm ơn ạ
Các bn giúp mình giải hết Bt này nhé (mình đang cần gấp lắm) , mình sẽ chọn đúng cho các câu trả lời của mn ạ. 
\(2.a.Magie+Axitclohidric\rightarrow MagieClorua+Hidro\\ b.Mg+2HCl\rightarrow MgCl_2+H_2\\ c.m_{Mg}+m_{HCl}=m_{MgCl_2}+m_{H_2}\\ d.m_{HCl}=m_{MgCl_2}+m_{H_2}-m_{Mg}=47,5+1-12=36,5\left(g\right)\)
Đúng 3
Bình luận (0)
\(1.a.2Mg+O_2-^{t^o}\rightarrow2MgO\\ b.Fe+2AgNO_3\rightarrow Fe\left(NO_3\right)_2+2Ag\\ c.C_2H_4+3O_2-^{t^o}\rightarrow2CO_2+2H_2O\\ d.CuO+2HCl\rightarrow CuCl_2+H_2O\\ e.2Na+2H_2O\rightarrow2NaOH+H_2\\ f.4Al+3O_2-^{t^o}\rightarrow2Al_2O_3\)
Đúng 3
Bình luận (0)












