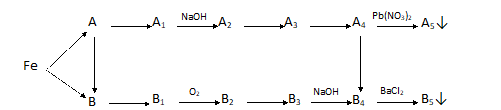Bài 1:
\(n_{Na}=\dfrac{8,05}{23}=0,35\left(mol\right)\); \(n_{AlCl_3}=0,2.0,5=0,1\left(mol\right)\)
PTHH: \(2Na+2H_2O\rightarrow2NaOH+H_2\)
0,35----------->0,35
\(AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3\downarrow+3NaCl\)
0,1----->0,3-------->0,1
\(Al\left(OH\right)_3+NaOH\rightarrow NaAlO_2+2H_2O\)
0,05<----0,05
\(\Rightarrow n_{Al\left(OH\right)_3}=0,1-0,05=0,05\left(mol\right)\Rightarrow m_{Al\left(OH\right)_3}=0,05.78=3,9\left(g\right)\)
Bài 2:
\(n_{NaOH}=1,3.0,5=0,65\left(mol\right)\); \(\left\{{}\begin{matrix}n_{AlCl_3}=1.0,1=0,1\left(mol\right)\\n_{FeCl_3}=1.0,1=0,1\left(mol\right)\end{matrix}\right.\)
PTHH: \(3NaOH+AlCl_3\rightarrow Al\left(OH\right)_3\downarrow+3NaCl\)
0,3<-----0,1-------->0,1
\(3NaOH+FeCl_3\rightarrow Fe\left(OH\right)_3\downarrow+3NaCl\)
0,3<-----0,1-------->0,1
\(NaOH+Al\left(OH\right)_3\rightarrow NaAlO_2+2H_2O\)
0,05-->0,05
=> Kết tủa gồm \(\left\{{}\begin{matrix}Al\left(OH\right)_3:0,1-0,05=0,05\left(mol\right)\\Fe\left(OH\right)_3:0,1\left(mol\right)\end{matrix}\right.\)
PTHH: 2Al(OH)3 --to--> Al2O3 + 3H2O
0,05-------->0,025
2Fe(OH)3 --to--> Fe2O3 + 3H2O
0,1----------->0,05
=> x = 0,025.102 + 0,05.160 = 10,55 (g)

 Help me :
Help me :