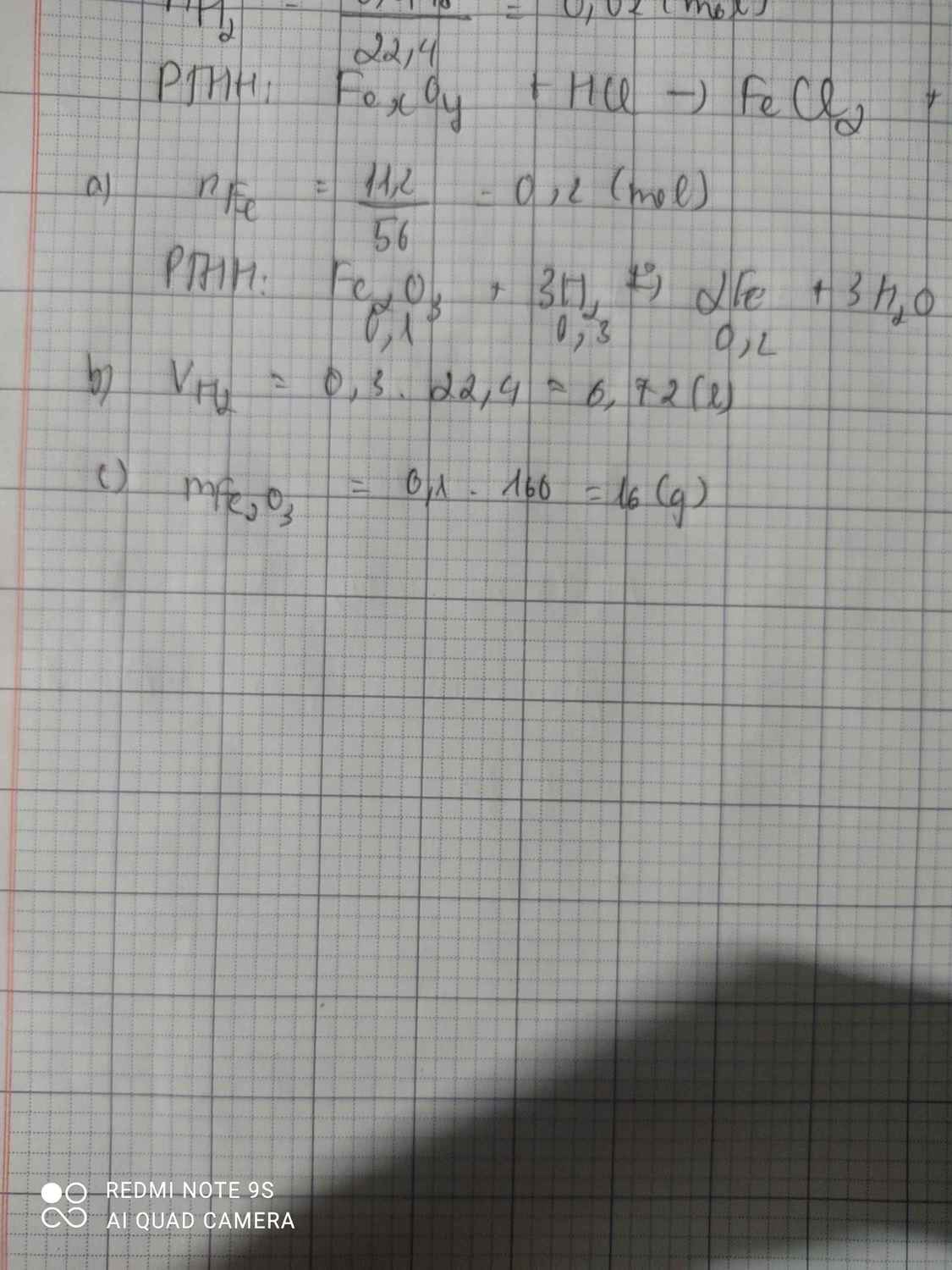a) Fe2O3 + 3H2 --to--> 2Fe + 3H2O
b) \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PTHH: Fe2O3 + 3H2 --to--> 2Fe + 3H2O
0,1<---0,3<--------0,2
=> \(V_{H_2}=0,3.22,4=6,72\left(l\right)\)
c) \(m_{Fe_2O_3}=0,1.160=16\left(g\right)\)
\(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\\ a,PTHH:3H_2+Fe_2O_3\rightarrow\left(t^o\right)2Fe+3H_2O\\ b,n_{H_2}=\dfrac{3}{2}.0,2=0,3\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\\ c,n_{Fe_2O_3}=\dfrac{1}{2}.0,2=0,1\left(mol\right)\\ m_{Fe_2O_3}=160.0,1=16\left(g\right)\)
a) \(n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
PTHH : 3H2 + Fe2O3 -> 2Fe + 3H2O
0,3 0,1 0,2
b. \(V_{H_2}=0,3.22,4=6,72\left(l\right)\)
c. \(m_{Fe_2O_3}=0,1.160=16\left(g\right)\)