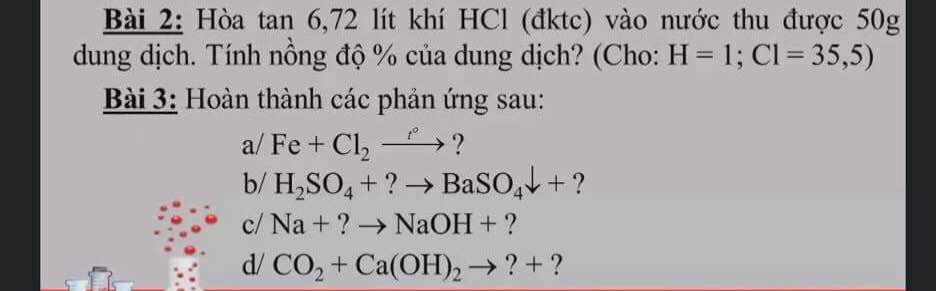
Bài 2 :
$n_{HCl} = \dfrac{6,72}{22,4} = 0,3(mol)$
$C\%_{HCl} = \dfrac{0,3.36,5}{50}.100\% = 21,9\%$
Bài 3 :
$a) 2Fe + 3Cl_2 \xrightarrow{t^o} 2FeCl_3$
$b) BaO + H_2SO_4 \to BaSO_4 + H_2O$
$c) 2Na + 2H_2O \to 2NaOH + H_2$
$d) CO_2 + Ca(OH)_2 \to CaCO_3 + H_2O$
Bài 2:
\(n_{HCl}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\Rightarrow m_{HCl}=0,3.36,5=10,95\left(g\right)\)
\(C\%_{ddHCl}=\dfrac{10,95.100\%}{50}=54,75\%\)
Bài 3:
\(a.2Fe+3Cl_2\underrightarrow{^{to}}2FeCl_3\\ b.Ba\left(OH\right)_2+H_2SO_4\rightarrow BaSO_4\downarrow+2H_2O\\ c.2Na+2H_2O\rightarrow2NaOH+H_2\uparrow\\ d.CO_2+Ca\left(OH\right)_2\rightarrow CaCO_3\downarrow+H_2O\)
Bài 2:
\(m_{HCl}=\dfrac{6,72}{22,4}.36,5=10,95\left(g\right)\\ C\%_{ddHCl}=\dfrac{10,95}{50}.100=21,9\%\)
HCl + H2O ---> H3O + Cl
Ta có: nHCl = \(\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
Theo PT: \(n_{H_3O}=n_{HCl}=0,3\left(mol\right)\)
=> \(m_{H_3O}=0,3.19=5,7\left(g\right)\)
=> C% = \(\dfrac{5,7}{50}.100\%=11,4\%\)
(Mik làm ở phần ảnh nha)
Bài 3:
a. 2Fe + 3Cl2 ---to---> 2FeCl3.
b. H2SO4 + Ba(OH)2 ---> BaSO4↓ + 2H2O
c. 2Na + 2H2O ---> 2NaOH + H2.
d. CO2 + Ca(OH)2 ---> CaCO3 + H2O