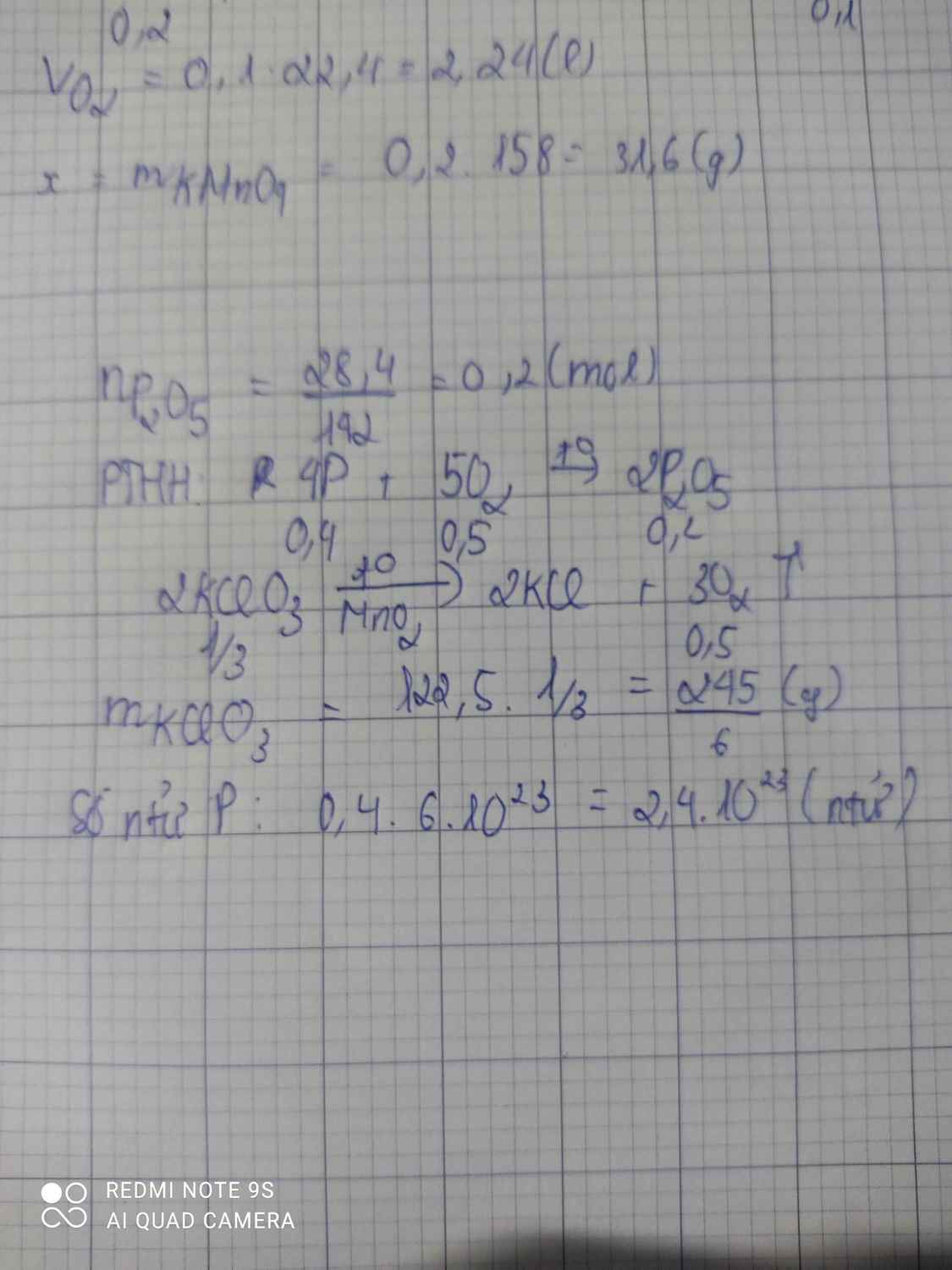2KClO3-to>2KCl+3O2
\(\dfrac{1}{3}\)------------------------0,5 mol
4P+5O2-to>2P2O5
0,4--0,5-------0,2 mol
n P2O5=\(\dfrac{28,4}{142}\)=0,2 mol
=>m KClO3=\(\dfrac{1}{3}\).122,5=40,83g
=> số nt P là :0,4.6.1023=2,4.1023
\(a,2KClO_3\rightarrow\left(t^o\right)2KCl+3O_2\\ 4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\\ b,n_{P_2O_5}=\dfrac{28,4}{142}=0,2\left(mol\right)\\ n_{O_2}=\dfrac{5}{2}.0,2=0,5\left(mol\right)\\ n_{KClO_3}=\dfrac{2}{3}.0,5=\dfrac{1}{3}\left(mol\right)\\ \Rightarrow m_{KClO_3}=\dfrac{122,5}{3}=\dfrac{245}{6}\left(g\right)\\ c,n_P=\dfrac{4}{2}.n_{P_2O_5}=2.0,2=0,4\left(mol\right)\\ \Rightarrow m_P=31.0,4=12,4\left(g\right)\)