\(Đặt.CTTQ.kim.loại:R\\ R+2H_2O\rightarrow R\left(OH\right)_2+H_2\\ n_{H_2}=\dfrac{0,168}{22,4}=0,0075\left(mol\right)\\ n_R=n_{H_2}=0,0075\left(mol\right)\\ M_R=\dfrac{0,3}{0,0075}=40\left(\dfrac{g}{mol}\right)\\ \Rightarrow R\left(II\right):Canxi\left(Ca=40\right)\)
Đúng 2
Bình luận (2)
Các câu hỏi tương tự
giải giúp mình bài 7 sgk/99 hóa 8 với ạ
Giải giúp mình bài này với! Mình cảm ơn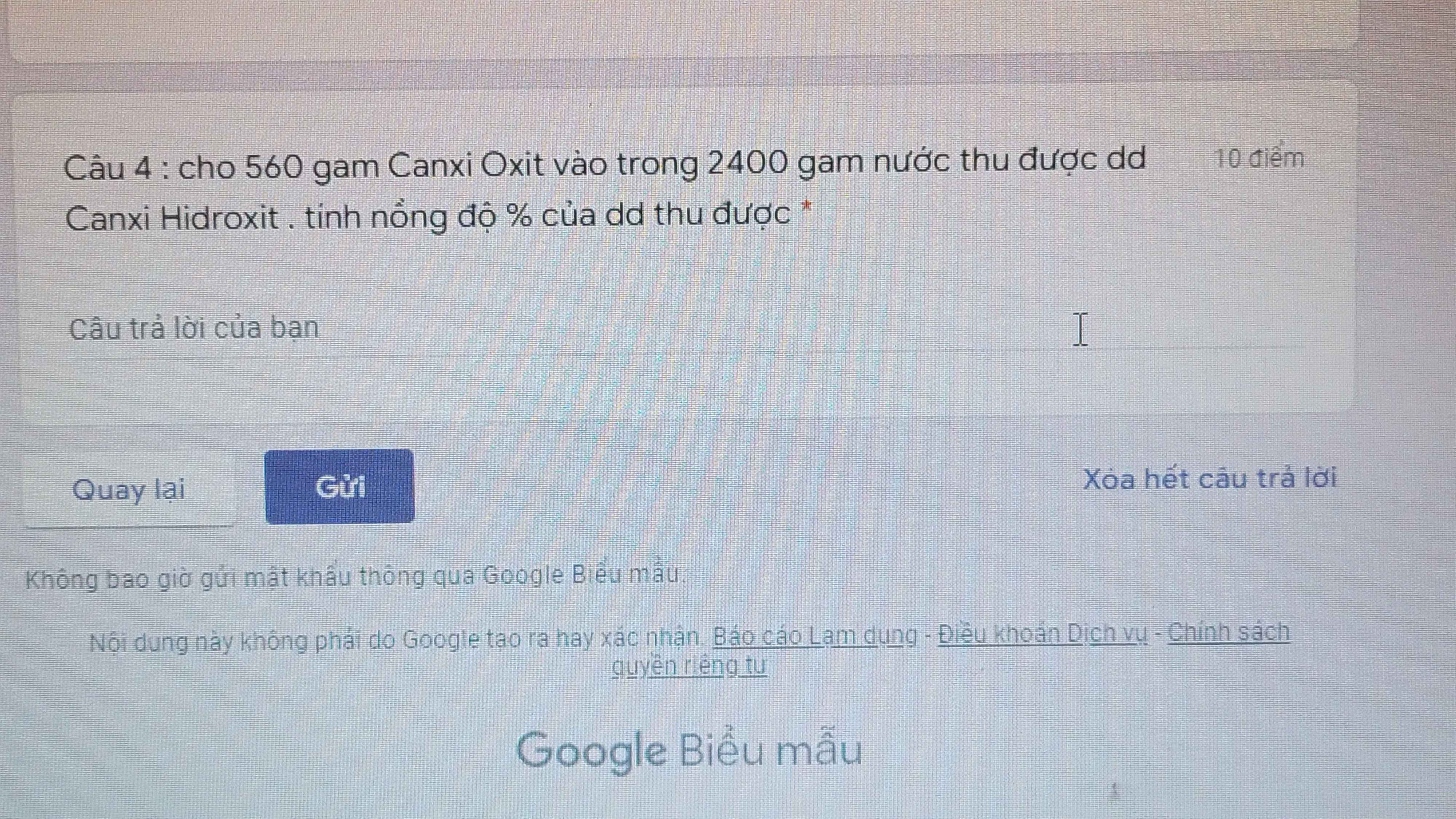
Giải CHI TIẾT giúp mình với, ảnh bài tập dưới phần bl nhé
Giải CHI TIẾT giúp mình với, ảnh bài tập dưới phần bl nhé
Giải chi tiết giúp mình với, ảnh bài tập dưới phần bl nhé
Giải chi tiết giúp mình với, ảnh bài tập dưới phần bl nhé
Giải chi tiết giúp mình với, ảnh bài tập dưới phần bl nhé
Giải chi tiết giúp mình với, ảnh bài tập dưới phần bl nhé
Giải chi tiết giúp mình với, ảnh bài tập dưới phần bl nhé






