\(n_S=\dfrac{m_S}{M_S}=\dfrac{8}{32}=0,25mol\)
\(n_{O_2}=\dfrac{m_{O_2}}{M_{O_2}}=\dfrac{7}{32}=\dfrac{7}{32}mol\)
\(S+O_2\rightarrow\left(t^o\right)SO_2\)
0,25 > 7/32 ( mol )
7/32 7/32 ( mol )
=> Chất dư là S
\(m_{S\left(dư\right)}=n_{S\left(dư\right)}.M_S=\left(0,25-\dfrac{7}{32}\right).32=1g\)
=> Chọn A


















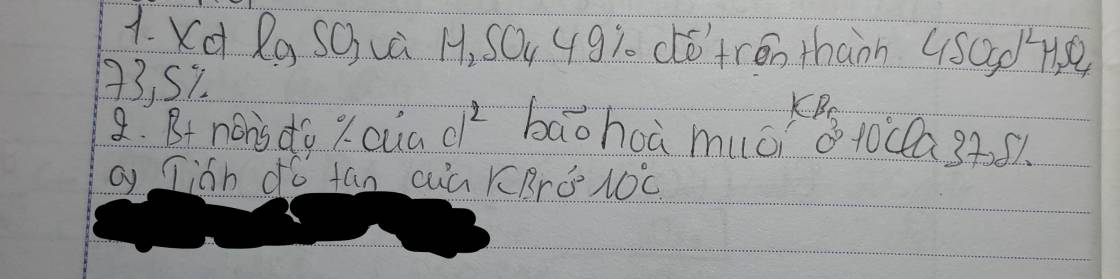 giải giúp mik vs ạ mik đang cần gấp!!!
giải giúp mik vs ạ mik đang cần gấp!!!
