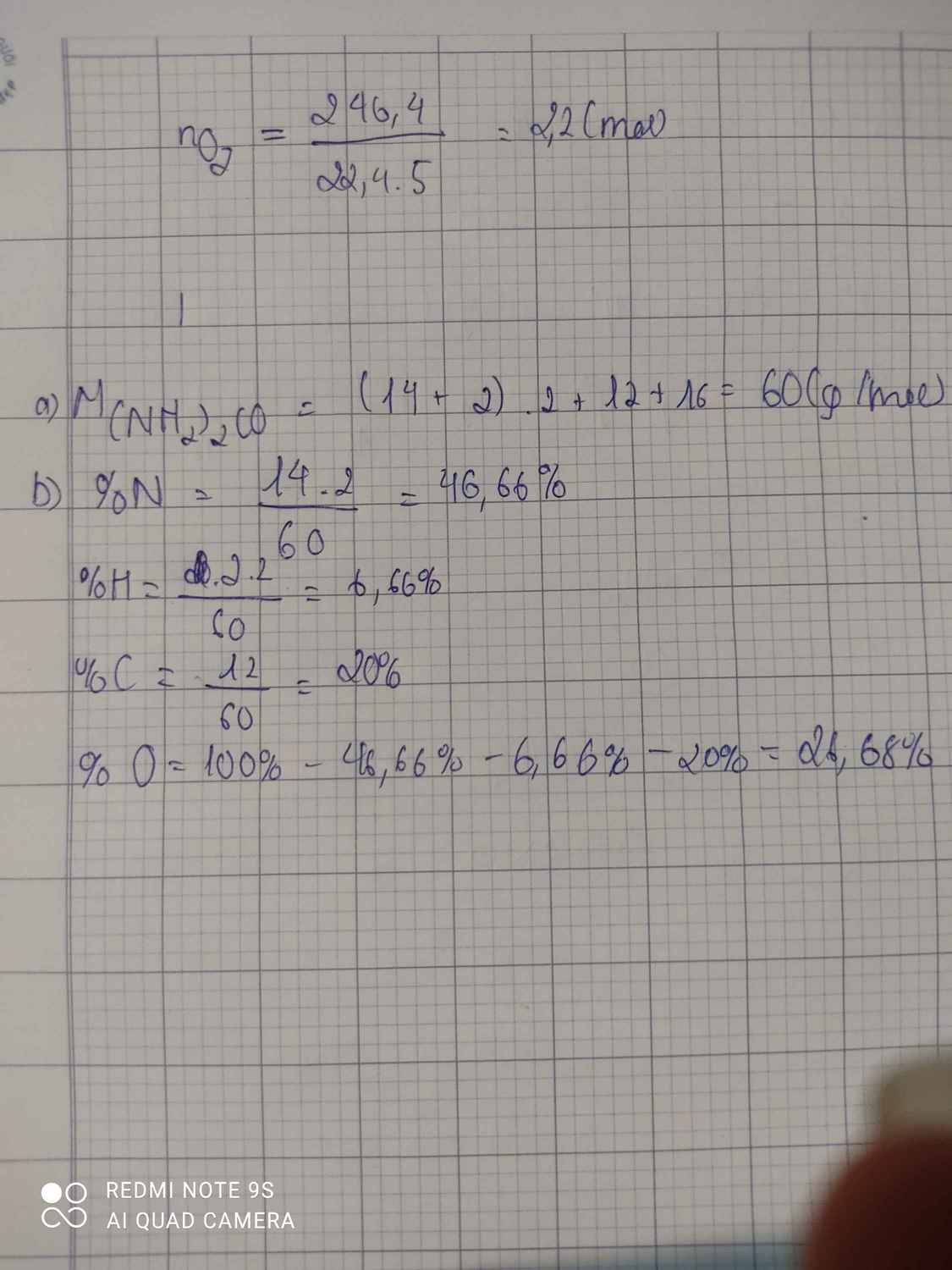a) \(M_{\left(NH_2\right)_2CO}=\left(14.1+1.2\right).2+12.1+16.1=60\left(g/mol\right)\)
b) \(\left\{{}\begin{matrix}\%N=\dfrac{2.14}{60}.100\%=46,67\%\\\%H=\dfrac{1.4}{60}.100\%=6,67\%\\\%C=\dfrac{12.1}{60}.100\%=20\%\\\%O=100\%-46,67\%-6,67\%-20\%=26,66\%\end{matrix}\right.\)