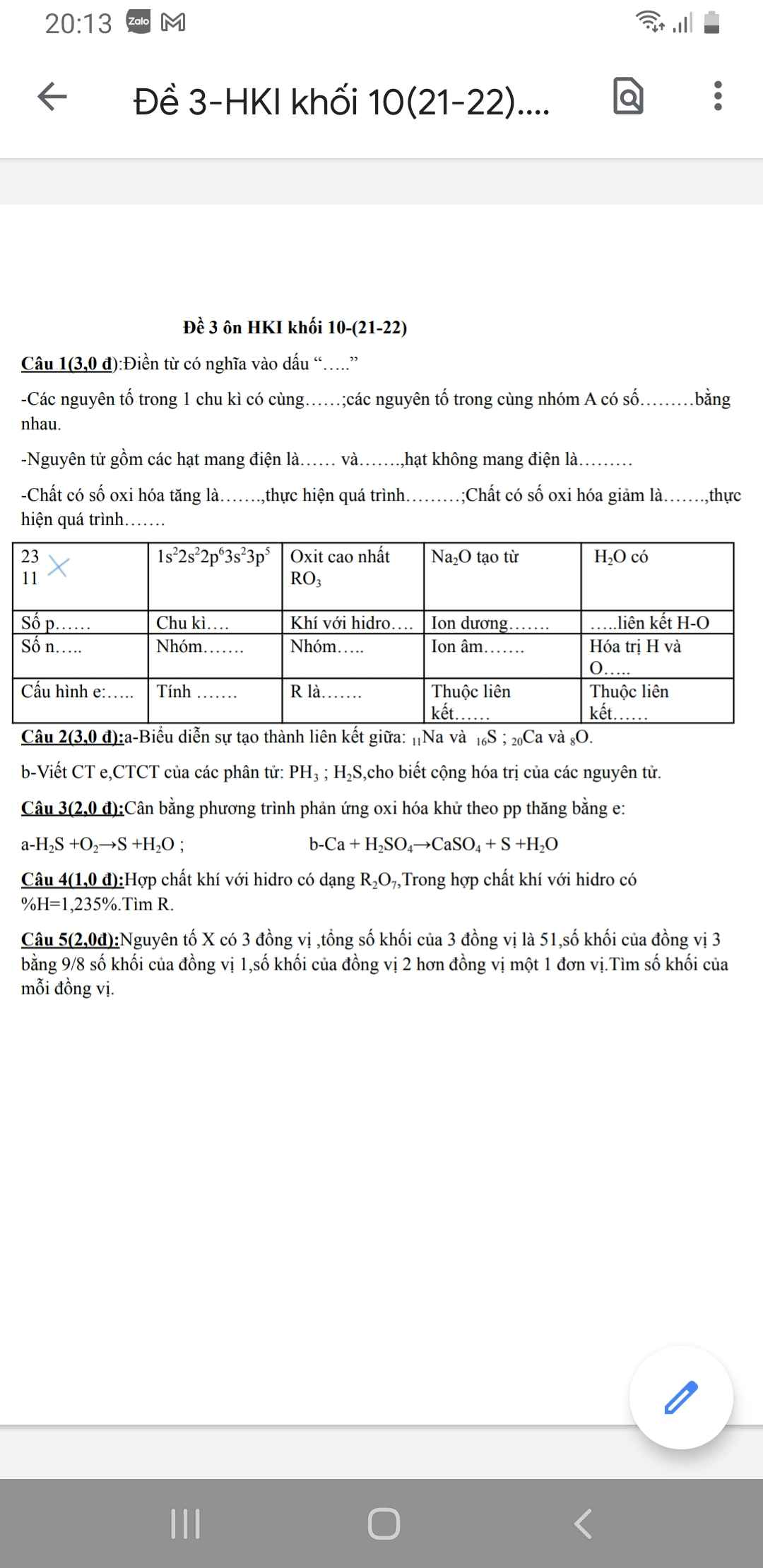
Bài 29 :
\(n_{CO2}=\dfrac{4,48}{22,4}=0,2\left(mol\right)\)
Pt : \(CaO+2HCl\rightarrow CaCl_2+H_2O|\)
1 2 1 1
0,3 0,6 0,3
\(CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O|\)
1 2 1 1 1
0,2 0,4 0,2 0,2
a) \(n_{CaCO3}=\dfrac{0,2.1}{1}=0,2\left(mol\right)\)
\(m_{CaCO3}=0,2.100=20\left(g\right)\)
\(m_{CaO}=36,8-20=16,8\left(g\right)\)
0/0CaO = \(\dfrac{16,8.100}{36,8}=45,65\)0/0
0/0CaCO3 = \(\dfrac{20.100}{36,8}=54,35\)0/0
b) Có : \(m_{CaO}=\dfrac{16,8}{56}=0,3\left(mol\right)\)
\(n_{HCl\left(tổng\right)}=0,6+0,4=1\left(mol\right)\)
\(C_{M_{ddHCl}}=\dfrac{1}{5}=0,2\left(M\right)\)
\(n_{CaCl2\left(tổng\right)}=0,3+0,2=0,5\left(mol\right)\)
\(C_{M_{CaCl2}}=\dfrac{0,5}{5}=0,1\left(M\right)\)
Chúc bạn học tốt
Bài 28:
\(CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\\ K_2CO_3+2HCl\rightarrow2KCl+CO_2+H_2O\\ Đặt:n_{CaCO_3}=a\left(mol\right);n_{K_2CO_3}=b\left(mol\right)\left(a,b>0\right)\\ \Rightarrow\left\{{}\begin{matrix}100a+138b=6,76\\a+b=\dfrac{2,64}{44}=0,06\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}a=0,04\\b=0,02\end{matrix}\right.\\ \%m_{CaCO_3}=\dfrac{0,02.100}{6,76}.100\approx29,586\%\\ \Rightarrow\%m_{K_2CO_3}\approx70,414\%\)