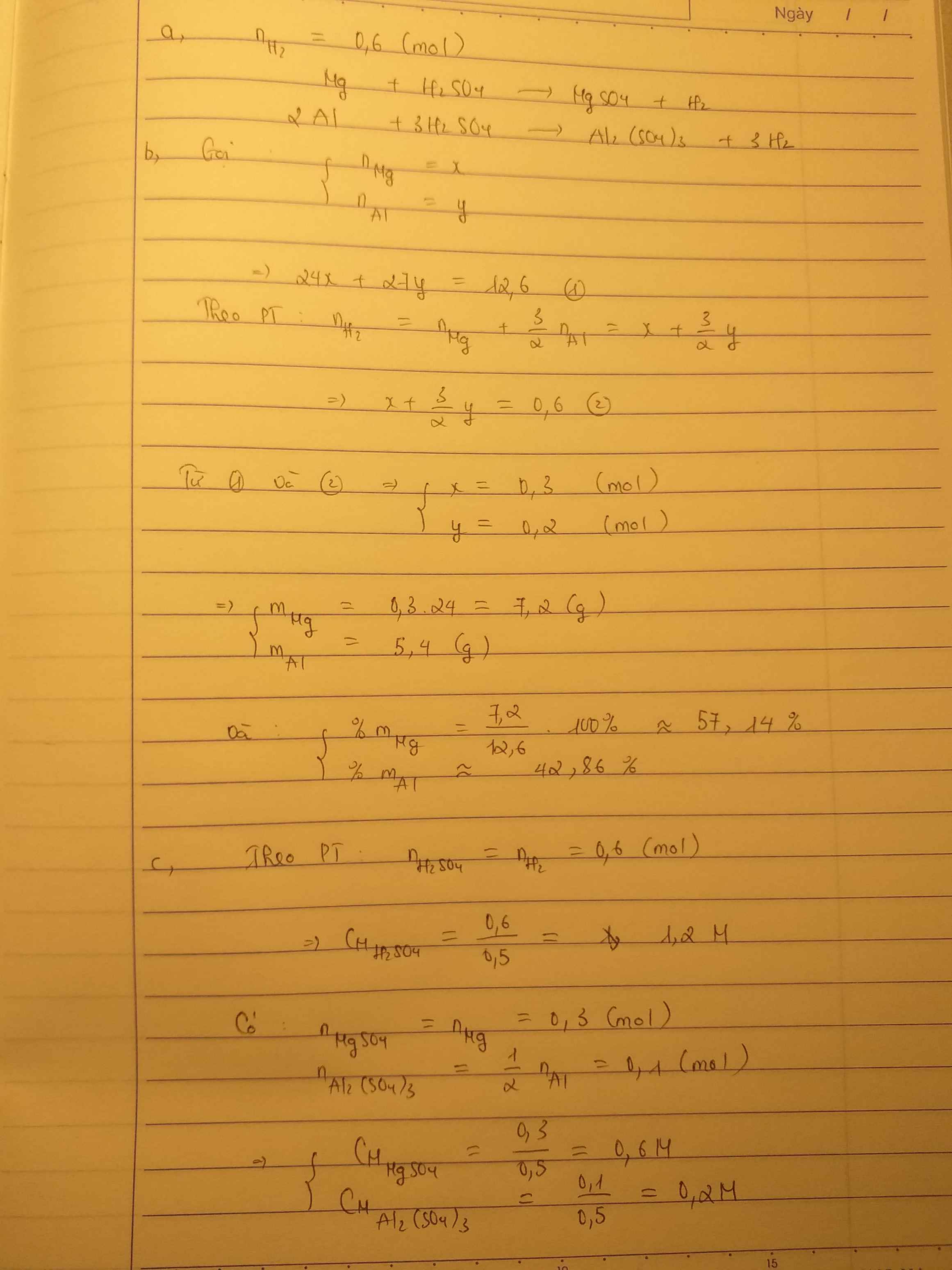a,\(n_{H_2}=\dfrac{13,44}{22,4}=0,6\left(mol\right)\)
PTHH: Mg + H2SO4 → MgSO4 + H2
Mol: x x
PTHH: 2Al + 3H2SO4 → Al2(SO4)3 + 3H2
Mol: y 1,5y
Ta có: \(\left\{{}\begin{matrix}24x+27y=12,6\\x+1,5y=0,6\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}x=0,3\\y=0,2\end{matrix}\right.\)
b,\(\%m_{Mg}=\dfrac{0,3.24.100\%}{12,6}=57,14\%;\%m_{Al}=100-57,14=42,86\%\)
c,
PTHH: Mg + H2SO4 → MgSO4 + H2
Mol: 0,3 0,3 0,3
PTHH: 2Al + 3H2SO4 → Al2(SO4)3 + 3H2
Mol: 0,2 0,3 0,1
\(C_{M_{ddH_2SO_4}}=\dfrac{0,3+0,3}{0,5}=1,2M\)
\(C_{M_{ddMgSO_4}}=\dfrac{0,3}{0,5}=0,6M;C_{M_{ddAl_2\left(SO_4\right)_3}}=\dfrac{0,1}{0,5}=0,2M\)