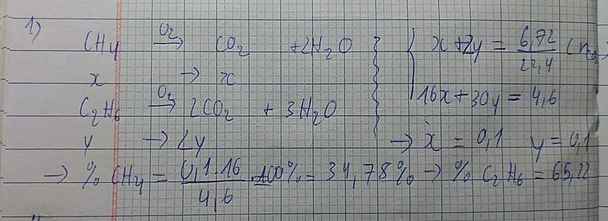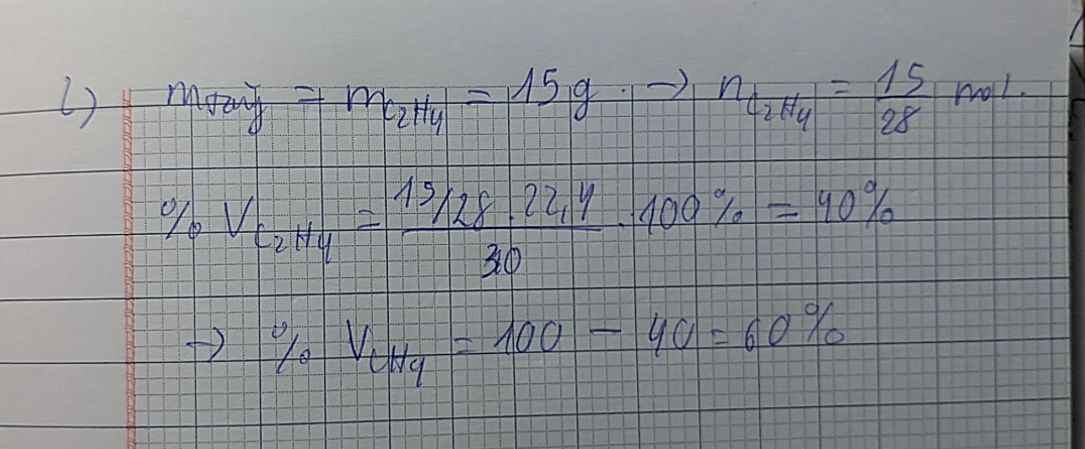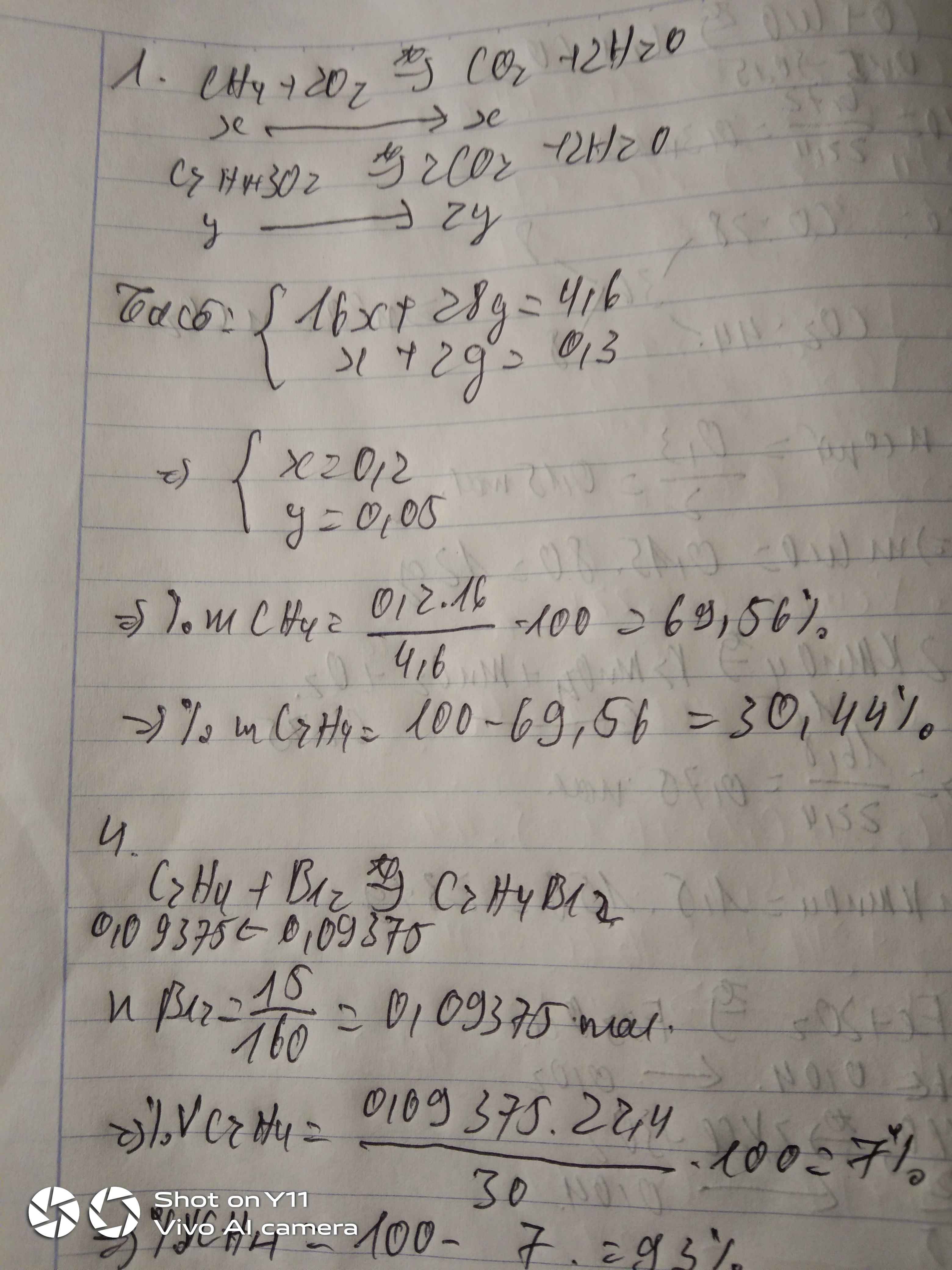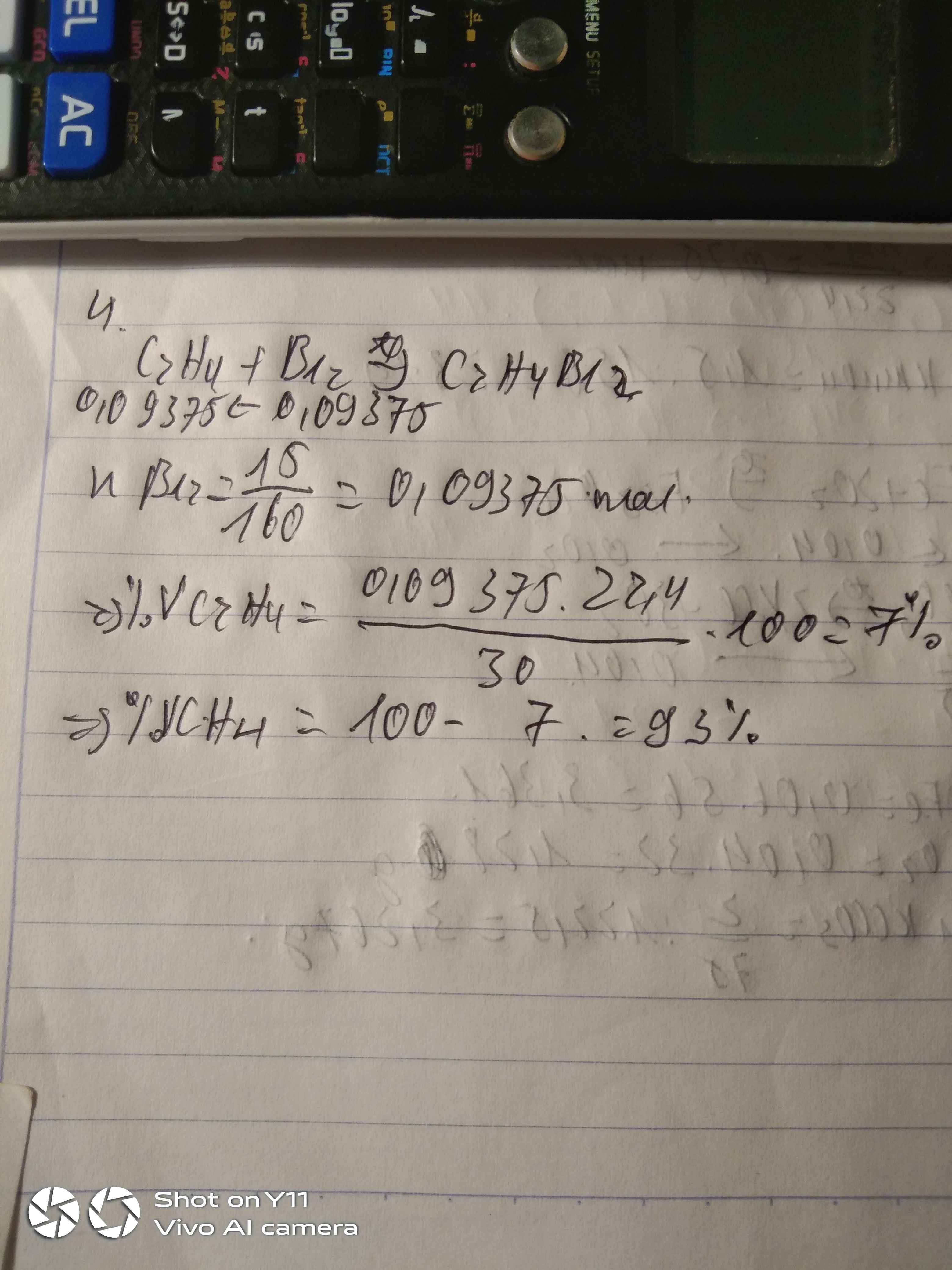Câu 1.
Gọi \(\left\{{}\begin{matrix}n_{CH_4}=a\left(mol\right)\\n_{C_2H_6}=b\left(mol\right)\end{matrix}\right.\)
\(n_{CO_2}=\dfrac{6,72}{22,4}=0,3mol\)
\(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_6+\dfrac{7}{2}O_2\underrightarrow{t^o}2CO_2+3H_2O\)
\(\Rightarrow\left\{{}\begin{matrix}16a+30b=4,6\\a+2b=0,3\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}a=0,1\\b=0,1\end{matrix}\right.\)
\(\%m_{CH_4}=\dfrac{0,1\cdot16}{4,6}\cdot100\%=34,78\%\)
\(\%m_{C_2H_6}=100\%-34,785=65,22\%\)