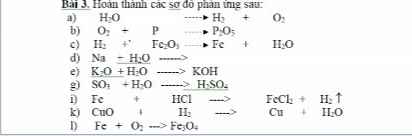
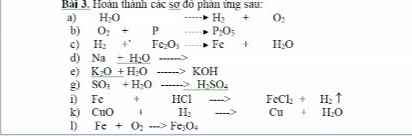
\(PTHH:CH_4+2O_2\underrightarrow{t^o}2H_2O+CO_2\)
\(n_{CH_4}=\dfrac{V_{\left(đktc\right)}}{22,4}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
\(Theo.PTHH:n_{O_2}=2.n_{CH_4}=2.0,05=0,1\left(mol\right)\\ V_{O_2\left(đktc\right)}=n.22,4=0,1.22,4=2,24\left(l\right)\\ \\ Theo.PTHH:n_{CO_2}=n_{CH_4}=0,05\left(mol\right)\\ V_{CO_2}=n.22,4=0,05.22,4=1,12\left(l\right)\)