1)
$CaCO_3 + 2HCl \to CaCl_2 + CO_2 + H_2O$
$CO_2 + 2NaOH \to Na_2CO_3 + H_2O$
2) $n_{CaCO_3} = \dfrac{35}{100} = 0,35(mol)$
$n_{HCl} = 2n_{CaCO_3} = 0,7(mol)$
$m_{HCl} = 0,7.36,5 = 25,55(gam)$
$m_{dd\ HCl} = \dfrac{25,55}{25\%} = 102,2(gam)$
3)
$n_{CO_2} = n_{CaCO_3} = 0,35(mol)$
$n_{NaOH} = 2n_{CO_2} = 0,7(mol)$
$\Rightarrow V_{dd\ NaOH} = \dfrac{0,7}{0,5} = 1,4(lít) = 1400(ml)$
1)
\(n_{CaCO_3}=\dfrac{35}{100}=0,35\left(mol\right)\)
PTHH: CaCO3 + 2HCl → CaCl2 + CO2 + H2O
Mol: 0,35 0,7 0,35
PTHH: CO2 + 2NaOH → Na2CO3 + H2O
Mol: 0,35 0,7
2) \(m_{ddHCl}=\dfrac{0,7.36,5.100}{25}=102,2\left(g\right)\)
3) \(V_{ddNaOH}=\dfrac{0,7}{0,5}=1,4\left(l\right)\)
Ta có: \(n_{CaCO_3}=\dfrac{35}{100}=0,35\left(mol\right)\)
1. PTHH:
CaCO3 + 2HCl ---> CaCl2 + CO2 + H2O (1)
CO2 + 2NaOH ---> Na2CO3 + H2O (2)
2. Theo PT(1): \(n_{HCl}=2.n_{CaCO_3}=2.0,35=0,7\left(mol\right)\)
=> \(m_{HCl}=0,7.36,5=25,55\left(g\right)\)
Ta có: \(C_{\%_{HCl}}=\dfrac{25,55}{m_{dd_{HCl}}}.100\%=25\%\)
=> \(m_{dd_{HCl}}=102,2\left(g\right)\)
3. Theo PT(1); \(n_{CO_2}=n_{CaCO_3}=0,35\left(mol\right)\)
Theo PT(2): \(n_{NaOH}=2.n_{CO_2}=2.0,35=0,7\left(mol\right)\)
=> \(V_{dd_{NaOH}}=\dfrac{0,7}{0,5}=1,4\left(lít\right)=1400\left(ml\right)\)










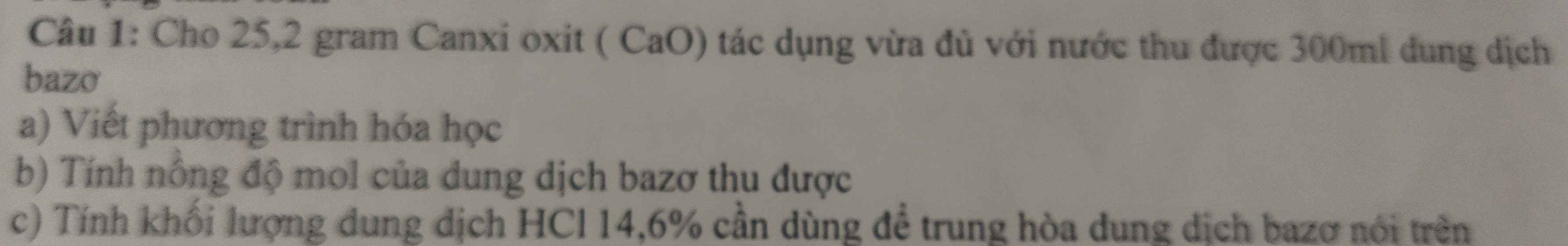











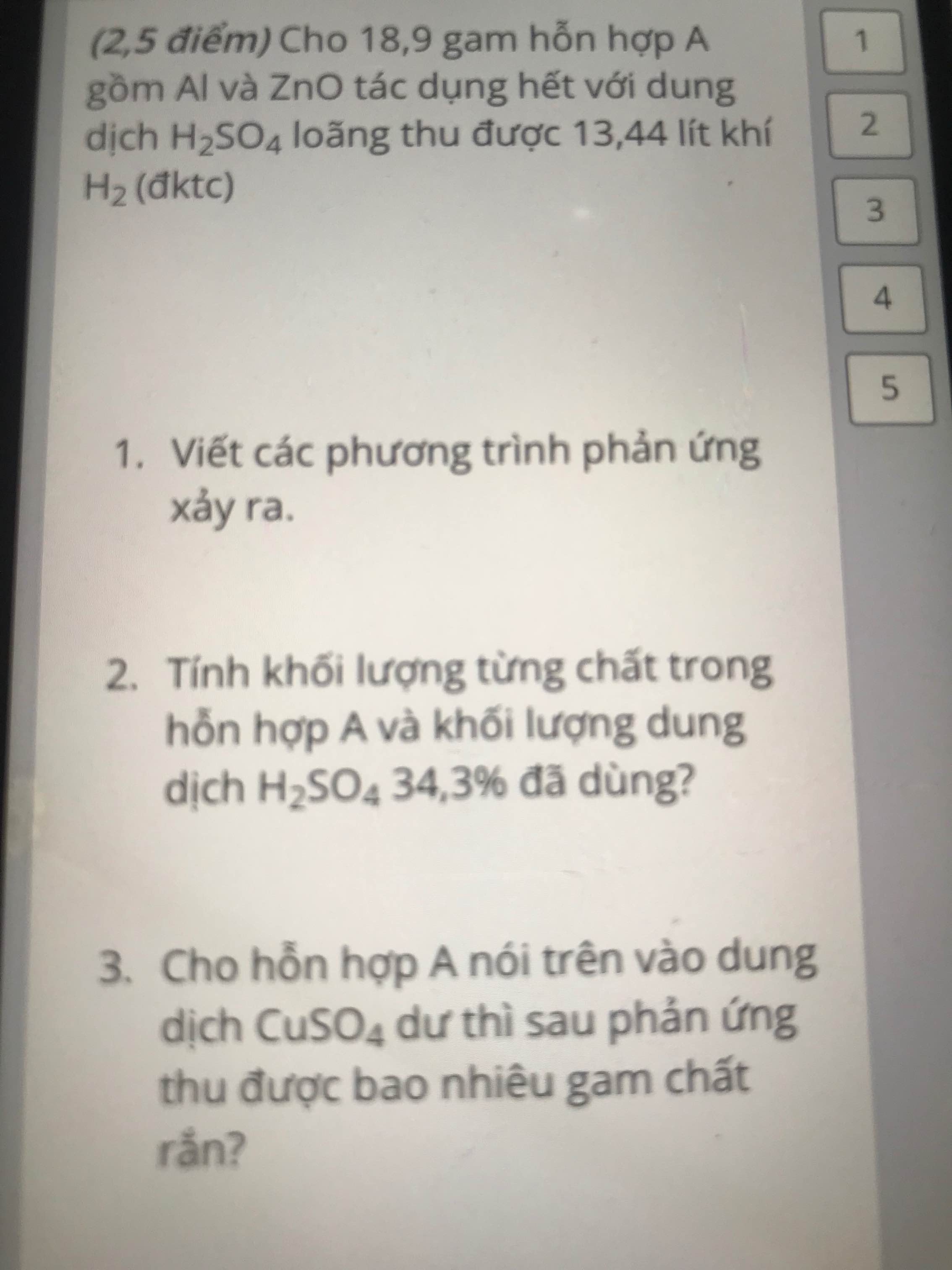
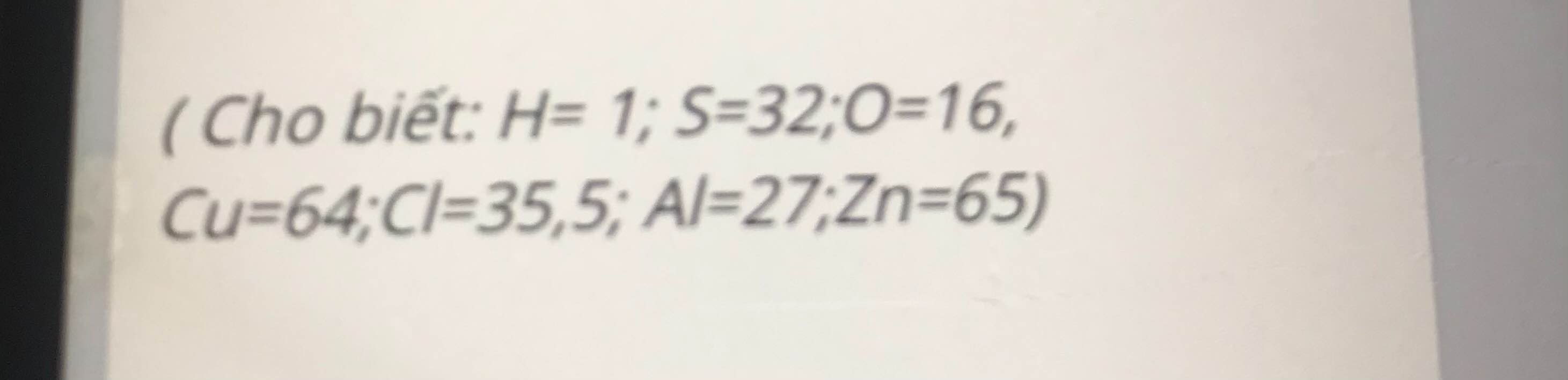

 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ
