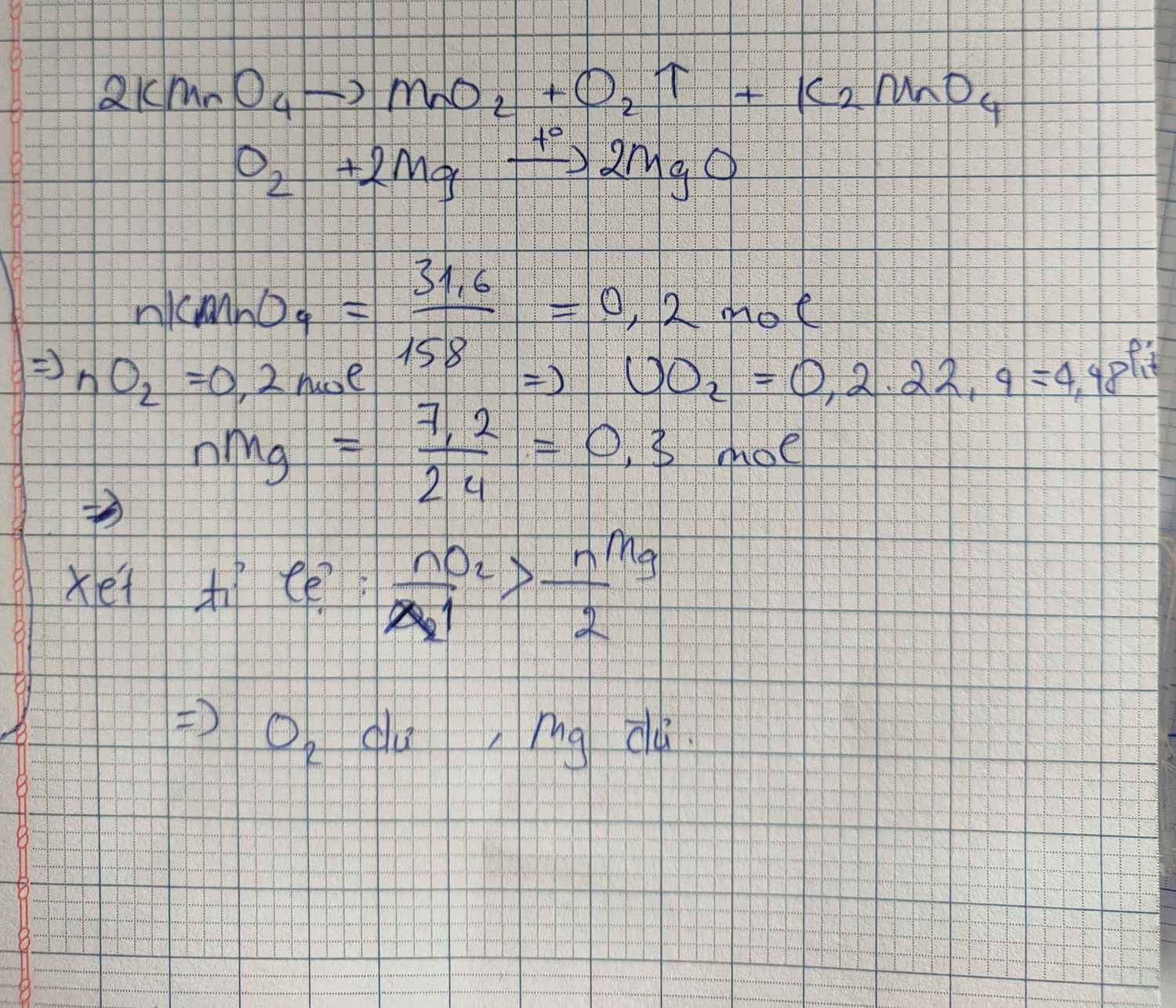a. \(2KMnO_4\underrightarrow{t^o}K_2MnO_4+MnO_2+O_2\)
0.2 0.1
\(n_{KMnO_4}=\dfrac{31.6}{158}=0.2mol\)
\(Mg+\dfrac{1}{2}O_2\underrightarrow{t^o}MgO\)
b. \(V_{O_2}=0.1\times22.4=2.24l\)
c. \(nMg=\dfrac{7.2}{24}=0.3mol\)
Ta có: \(\dfrac{0.3}{1}>\dfrac{0.1}{\dfrac{1}{2}}\)
Vậy Mg dư