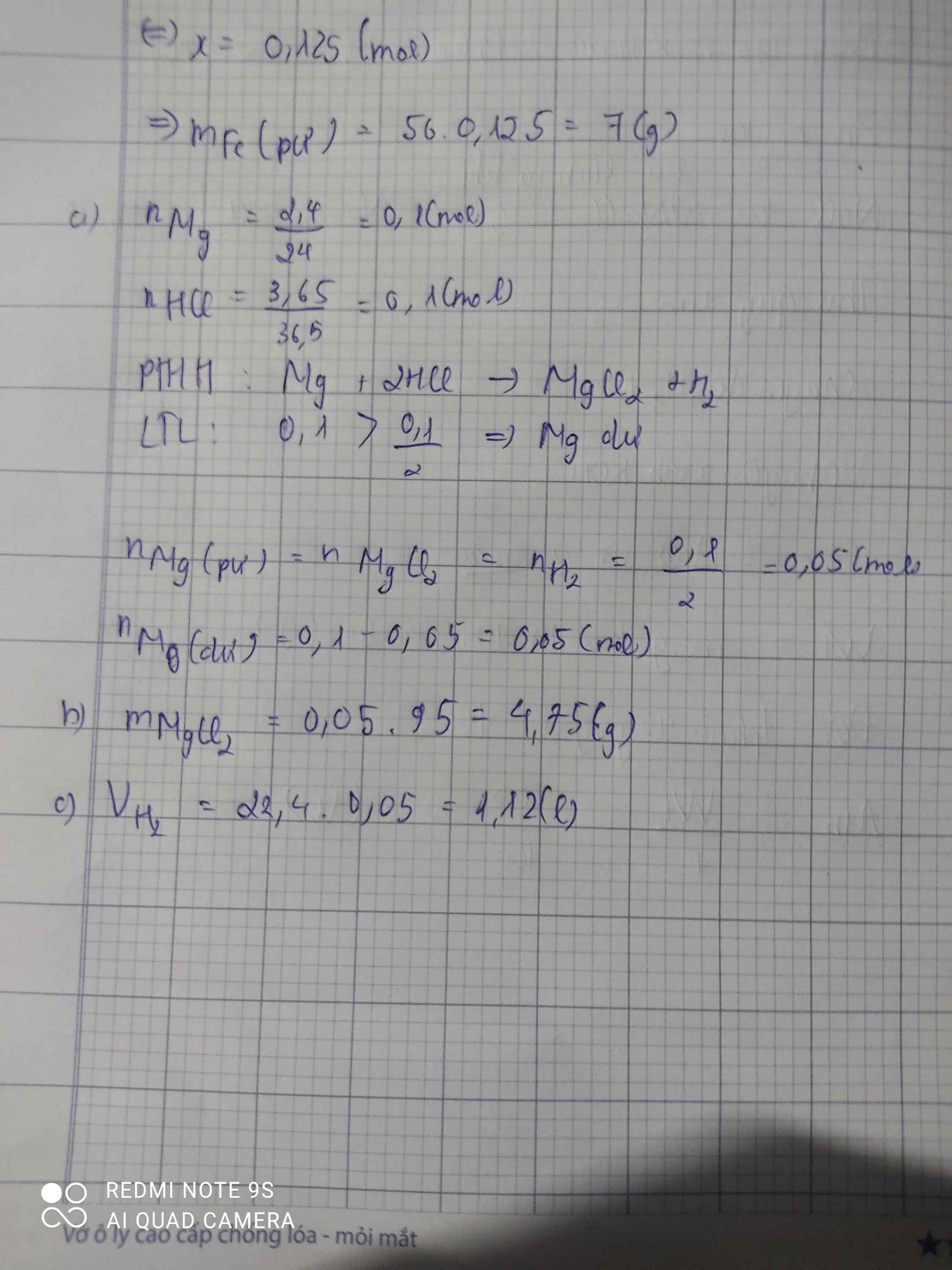\(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right);n_{HCl}=\dfrac{3,65}{36,5}=0,1\left(mol\right)\\ a,Mg+2HCl\rightarrow MgCl_2+H_2\\ Vì:\dfrac{0,1}{2}< \dfrac{0,1}{1}\\ \Rightarrow Mgdư\\ \Rightarrow n_{Mg\left(p.ứ\right)}=n_{MgCl_2}=n_{H_2}=\dfrac{0,1}{2}=0,05\left(mol\right)\\ n_{Mg\left(dư\right)}=0,1-0,05=0,05\left(mol\right)\\ \Rightarrow m_{Mg\left(dư\right)}=0,05.24=1,2\left(g\right)\\ b,m_{MgCl_2}=95.0,05=4,75\left(g\right)\\ c,V_{H_2\left(đktc\right)}=0,05.22,4=1,12\left(l\right)\)
a. \(n_{Mg}=\dfrac{2,4}{24}=0,1\left(mol\right)\)
\(n_{HCl}=\dfrac{3,65}{36,5}=0,1\left(mol\right)\)
PTHH : Mg + 2HCl -> MgCl2 + H2
0,05 0,1 0,05 0,05
Xét tỉ lệ : \(\dfrac{0,1}{1}>\dfrac{0,1}{2}\) => HCl đủ , Mg dư
\(n_{Mg\left(dư\right)}=0,1-0,05=0,05\left(mol\right)\)
b. \(m_{MgCl_2}=0,05.95=4,75\left(g\right)\)
c. \(V_{H_2}=0,05.22,4=1,12\left(l\right)\)