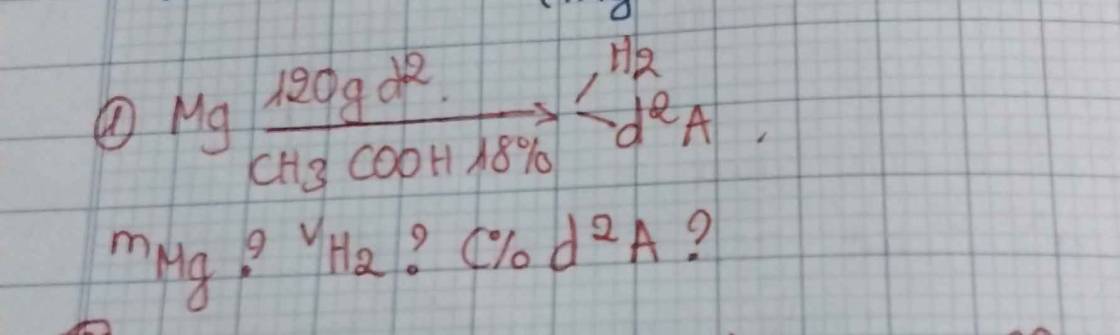
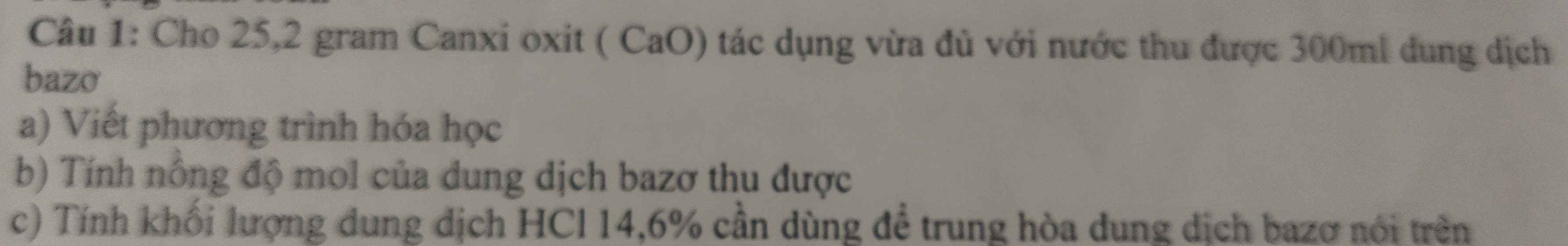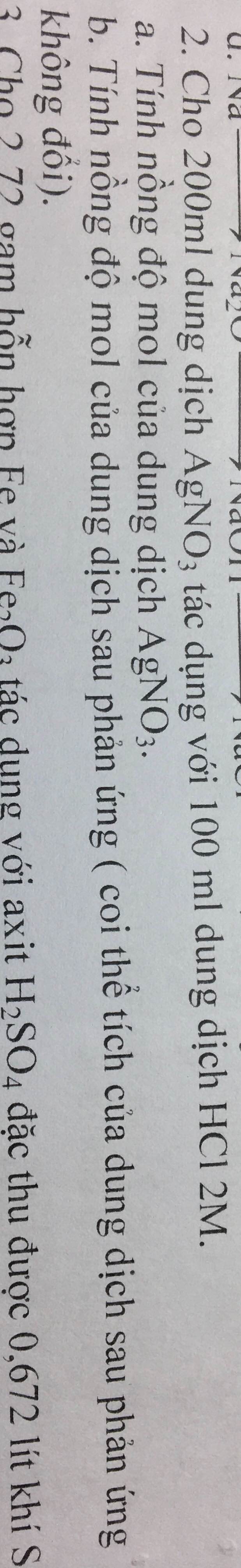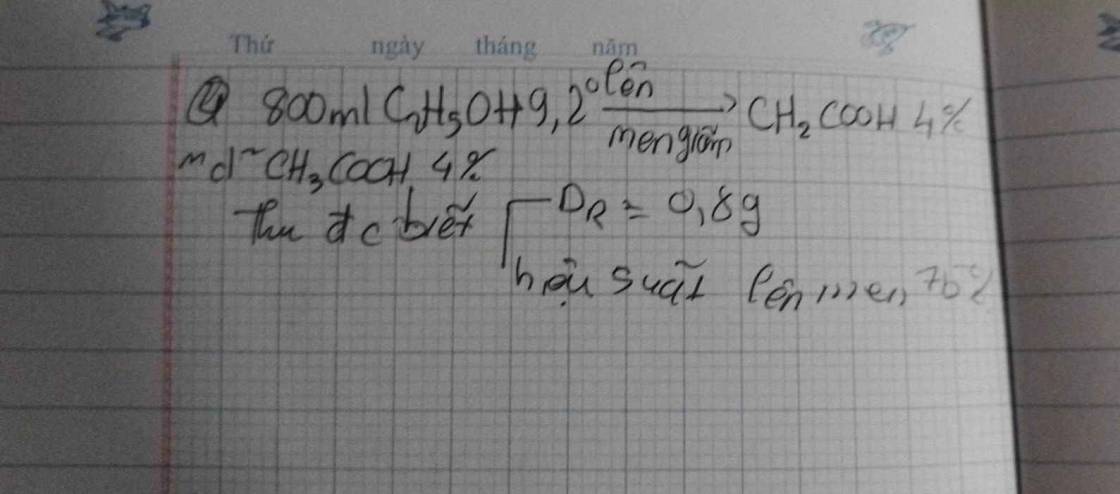a) PT: \(NaCl+AgNO_3\xrightarrow[]{}NaNO_3+AgCl\)
(mol) 0,04..........0,04...........0,04.............0,04
\(n_{NaCl}=\dfrac{2,34}{58,5}=0,04\left(mol\right)\)
\(m_{AgCl}=143,5.0,04=5,74\left(g\right)\)
b) \(m_{AgNO_3}=170.0,04=6,8\left(g\right)\)
c) Theo BTKL: \(m_{NaCl}+m_{ddAgNO_3}=m_{ddsau}+m_{AgCl}\)
⇒ \(m_{ddsau}=2,34+\dfrac{6,8.100}{3,4}-5,74=196,6\left(g\right)\)
\(C\%_{NaNO_3}=\dfrac{85.0,04}{196,6}.100=1,73\%\)