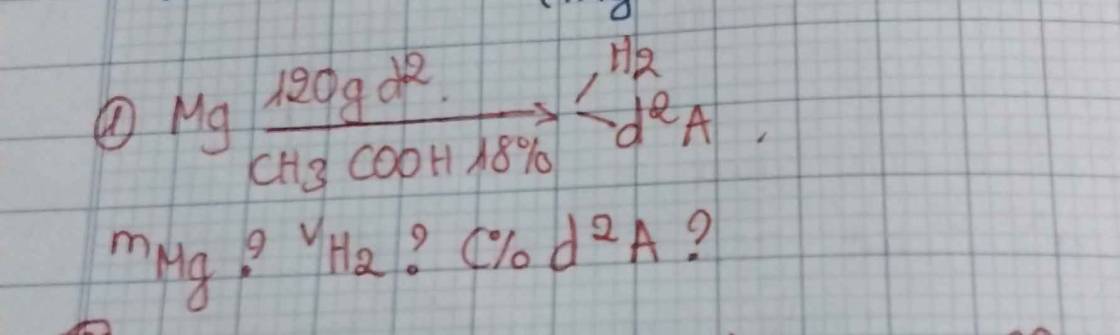
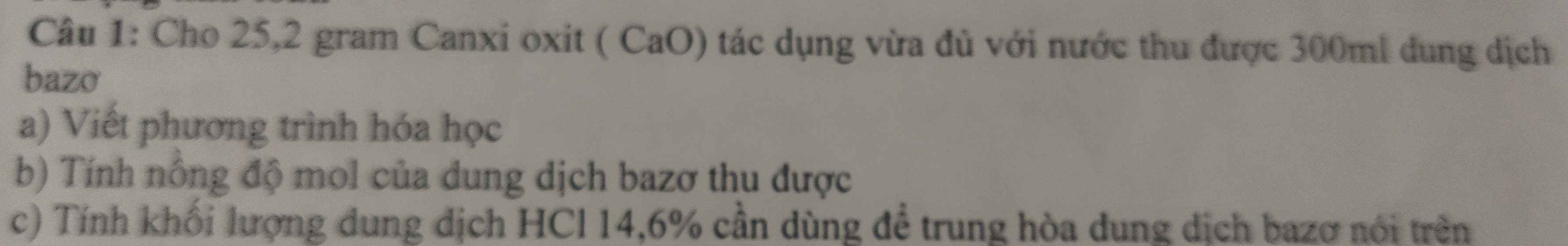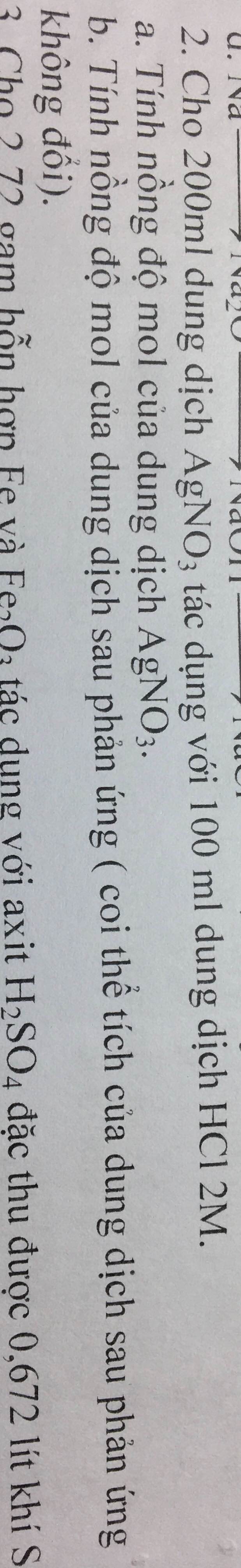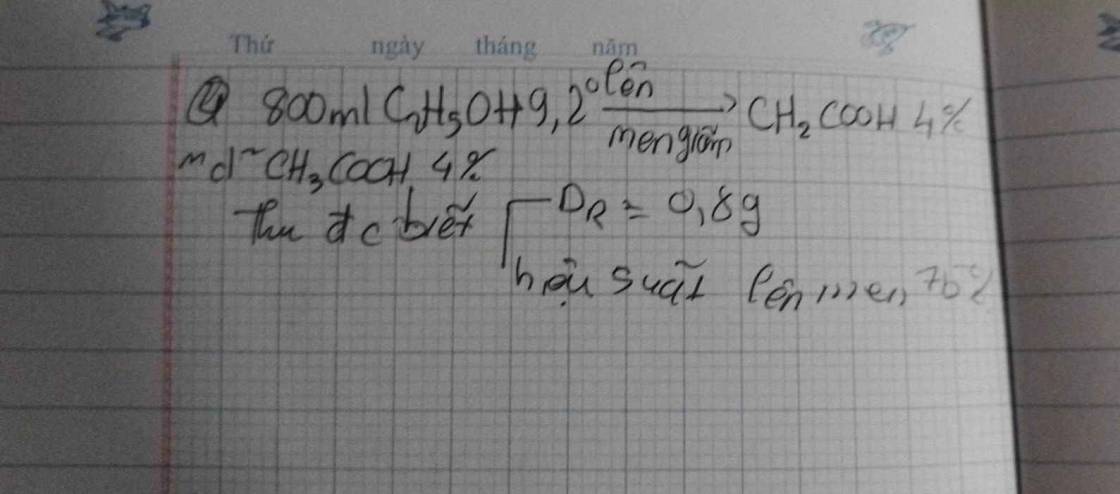\(C_2H_5OH+O_2\underrightarrow{mengiam}CH_3COOH+H_2O\)
a, Ta có: \(V_{C_2H_5OH}=\dfrac{8.10}{100}=0,8\left(l\right)=800\left(cm^3\right)\)
\(\Rightarrow m_{C_2H_5OH}=800.0,8=640\left(g\right)\Rightarrow n_{C_2H_5OH}=\dfrac{640}{46}=\dfrac{320}{23}\left(mol\right)\)
Theo PT: \(n_{CH_3COOH\left(LT\right)}=n_{C_2H_5OH}=\dfrac{320}{23}\left(mol\right)\)
Mà: H = 92% \(\Rightarrow n_{CH_3COOH\left(TT\right)}=\dfrac{320}{23}.92\%=12,8\left(mol\right)\)
\(\Rightarrow m_{CH_3COOH}=12,8.60=768\left(g\right)\)
b, Ta có: \(m_{ddgiam}=\dfrac{768}{4\%}=19200\left(g\right)\)