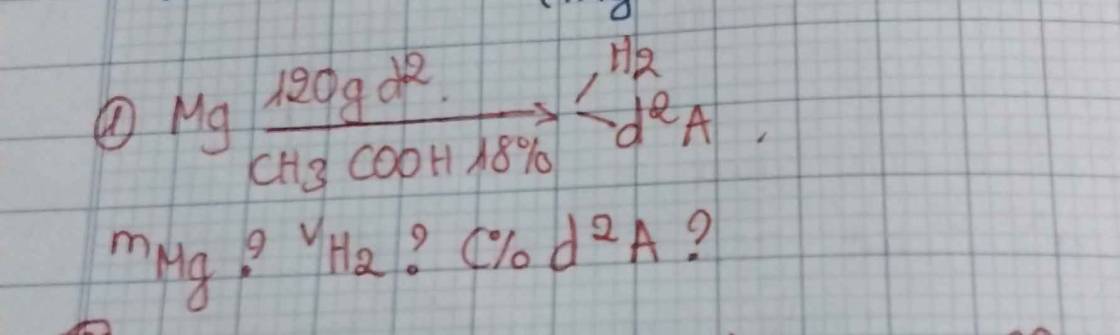
\(m_{CH_3COOH}=120.18\%=21,6\left(g\right)\Rightarrow n_{CH_3COOH}=\dfrac{21,6}{60}=0,36\left(mol\right)\)
PT: \(Mg+2CH_3COOH\rightarrow\left(CH_3COO\right)_2Mg+H_2\)
____0,18__________0,36___________0,18______0,18 (mol)
\(m_{Mg}=0,18.24=4,32\left(g\right)\)
\(V_{H_2}=0,18.22,4=4,032\left(l\right)\)
m dd sau pư = 4,32 + 120 - 0,18.2 = 123,96 (g)
\(\Rightarrow C\%_{\left(CH_3COO\right)_2Mg}=\dfrac{0,18.142}{123,96}.100\%\approx20,62\%\)