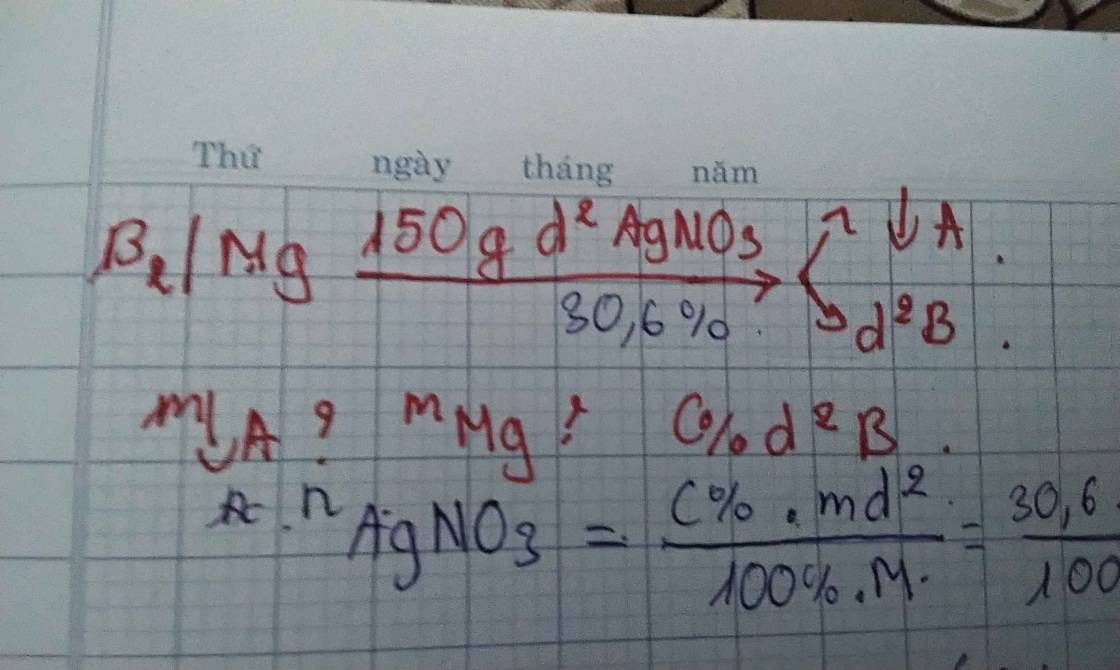PT: \(Mg+2AgNO_3\rightarrow Mg\left(NO_3\right)_2+2Ag_{\downarrow}\)
Ta có: \(n_{AgNO_3}=\dfrac{150.30,6\%}{170}=0,27\left(mol\right)\)
Theo PT: \(\left\{{}\begin{matrix}n_{Mg}=n_{Mg\left(NO_3\right)_2}=\dfrac{1}{2}n_{AgNO_3}=0,135\left(mol\right)\\n_{Ag}=n_{AgNO_3}=0,27\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow m_A=m_{Ag}=0,27.108=29,16\left(g\right)\)
\(m_{Mg}=0,135.24=3,24\left(g\right)\)
Ta có: m dd sau pư = mMg + m dd AgNO3 - mAg = 124,08 (g)
\(\Rightarrow C\%_{Mg\left(NO_3\right)_2}=\dfrac{0,135.148}{124,08}.100\%\approx16,1\%\)