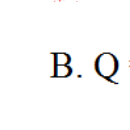\(n_{O_2}=\dfrac{m_{O_2}}{M_{O_2}}=\dfrac{6}{32}=0,1875mol\)
\(n_P=\dfrac{m_P}{M_P}=\dfrac{7}{31}=0,225mol\)
\(4P+5O_2\rightarrow\left(t^o\right)2P_2O_5\)
4 5 2 ( mol )
0,225 > 0,1875 ( mol )
0,15 0,1875 ( mol )
Sau phản ứng chất dư là Photpho
4P+5O2-to>P2O5
n O2=\(\dfrac{6}{32}\)=0,1875 mol
n P=\(\dfrac{7}{31}\)=0,2258 mol
P dư
A,
4P+5O2----> P2O5
B,
0,1875 0,075
nO2=0,1875mol
nP=0,3mol
0,3/4>0,1875/5
P>O2
P dư, O2 hết
mP2O5=0,075.142=10,65g
A, Photpho, 10,65g
4P + 5O2 ---> 2P2O5
nO2 = 0,1875 ( mol )
Ta thấy :
\(0,1875< \dfrac{7}{31}\)
=> Photpho dư