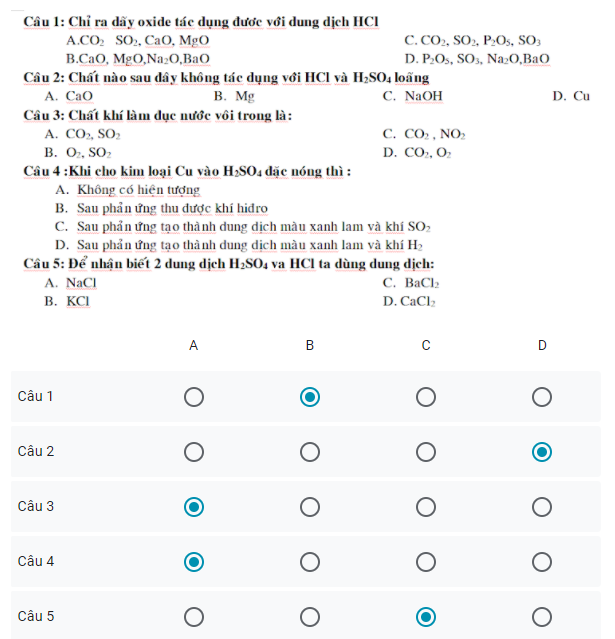
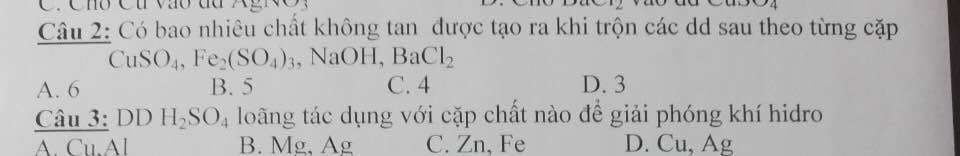Câu 12:
\(m_{KOH}=\dfrac{44,8\cdot25\%}{100\%}=11,2\left(g\right)\\ \Rightarrow n_{KOH}=\dfrac{11,2}{56}=0,2\left(mol\right)\\ PTHH:KOH+HCl\rightarrow KCl+H_2O\\ \Rightarrow n_{HCl}=0,2\left(mol\right)\\ \Rightarrow m_{CT_{HCl}}=0,2\cdot36,5=7,3\left(g\right)\\ \Rightarrow m_{dd_{HCl}}=\dfrac{7,3\cdot100\%}{2,5\%}=292\left(g\right)\\ \text{Chọn }C\)
Câu 13:
\(n_{HCl}=1\cdot0,5=0,5\left(mol\right)\\ PTHH:NaOH+HCl\rightarrow NaCl+H_2O\\ \Rightarrow n_{NaOH}=n_{HCl}=0,5\left(mol\right)\\ \Rightarrow V_{dd_{NaOH}}=\dfrac{0,5}{2}=0,25\left(l\right)=250\left(ml\right)\\ \text{Chọn B}\)