\(\left(1\right)FeCl_2+2NaOH\to Fe\left(OH\right)_2\downarrow +2NaCl\\ \left(2\right)Fe\left(OH\right)_2\xrightarrow{t^0}FeO+H_2O\\ \left(3\right)FeO+C\to Fe+CO\uparrow \\ \left(4\right)2Fe+3Cl_2\xrightarrow[]{t^o}2FeCl_3\\ \left(5\right)FeCl_3+3NaOH\to Fe\left(OH\right)_3\downarrow +3NaCl\\ \left(6\right)2Fe\left(OH\right)_3\xrightarrow{t^o}Fe_2O_3+3H_2O\\ \left(7\right)Fe_2O_3+3CO\xrightarrow{t^o}2Fe+3CO_2\\ \left(8\right)Fe+2HCl\to FeCl_2+H_2\)
Đúng 0
Bình luận (0)
Các câu hỏi tương tự
Giúp hộ e bài 8 với ạ, e đang cần gấp ạ! E cảm ơn trước ạ 
Cho 400g dd HCl 3,65%tác dụng vừa đủ với ddBa(OH)2 17,1%
a/ Tính m ddBa(OH)2 phản ứng ?
b/ Tính C% của dd thu được sau phản ứng ?
cứu mk vs ạ
Giúp e bài này với ạ,e đang cần gấp.E cảm ơn trước ạ!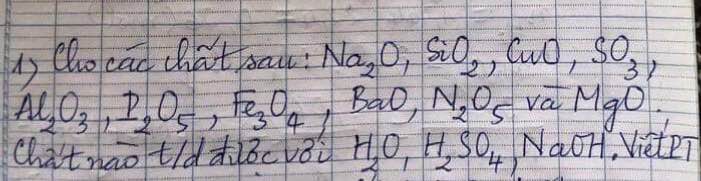
Cho 5,6g oxit kim loại hóa trị II tác dụng vừa đủ với V lít CO2 (dktc) thu được 10g muối. Tìm công thức hóa học của oxit.
Mong mn giúp e sớm nhất có thể với ạ, e đang cần gấp ạ. E cảm ơn.
Giúp e với ạ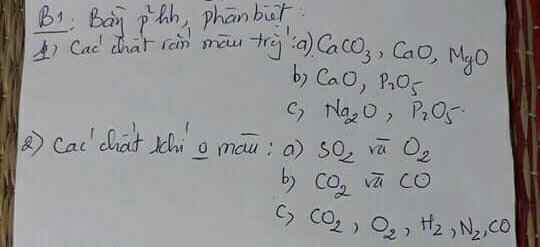
Giúp e với ạ
Giúp e với ạ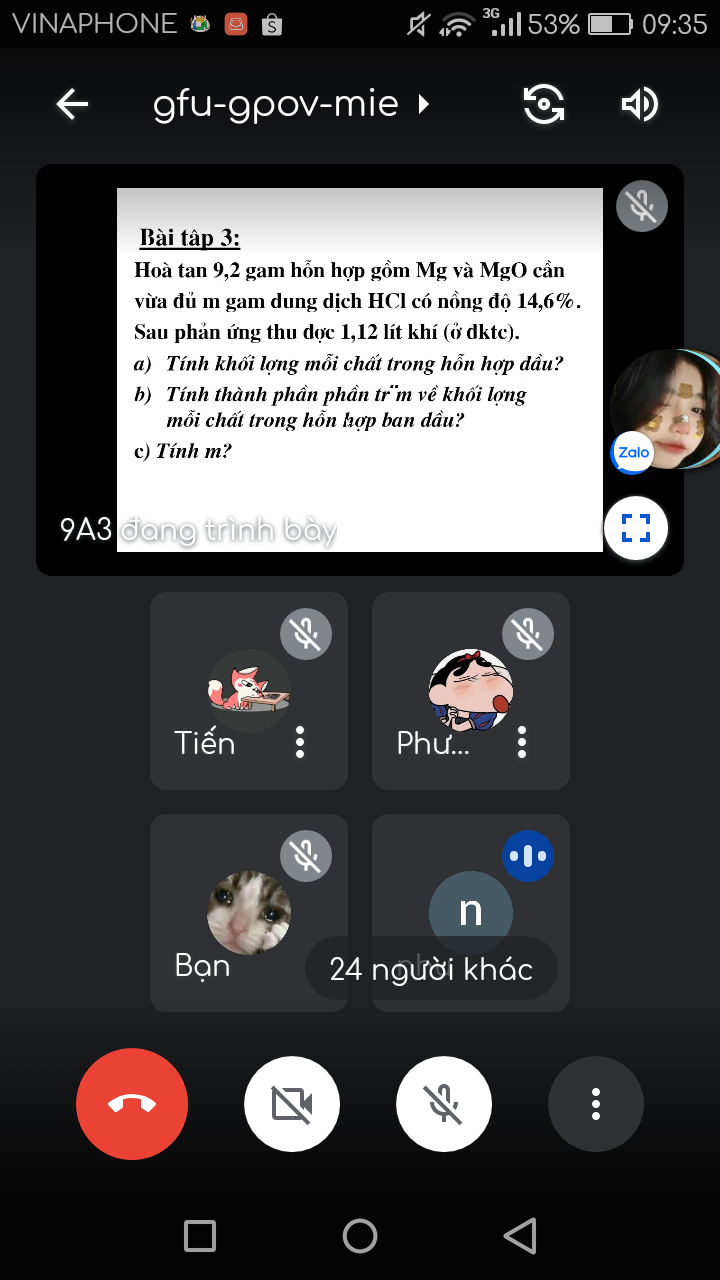
giúp e với ạ
 Giúp e với ạ
Giúp e với ạ












