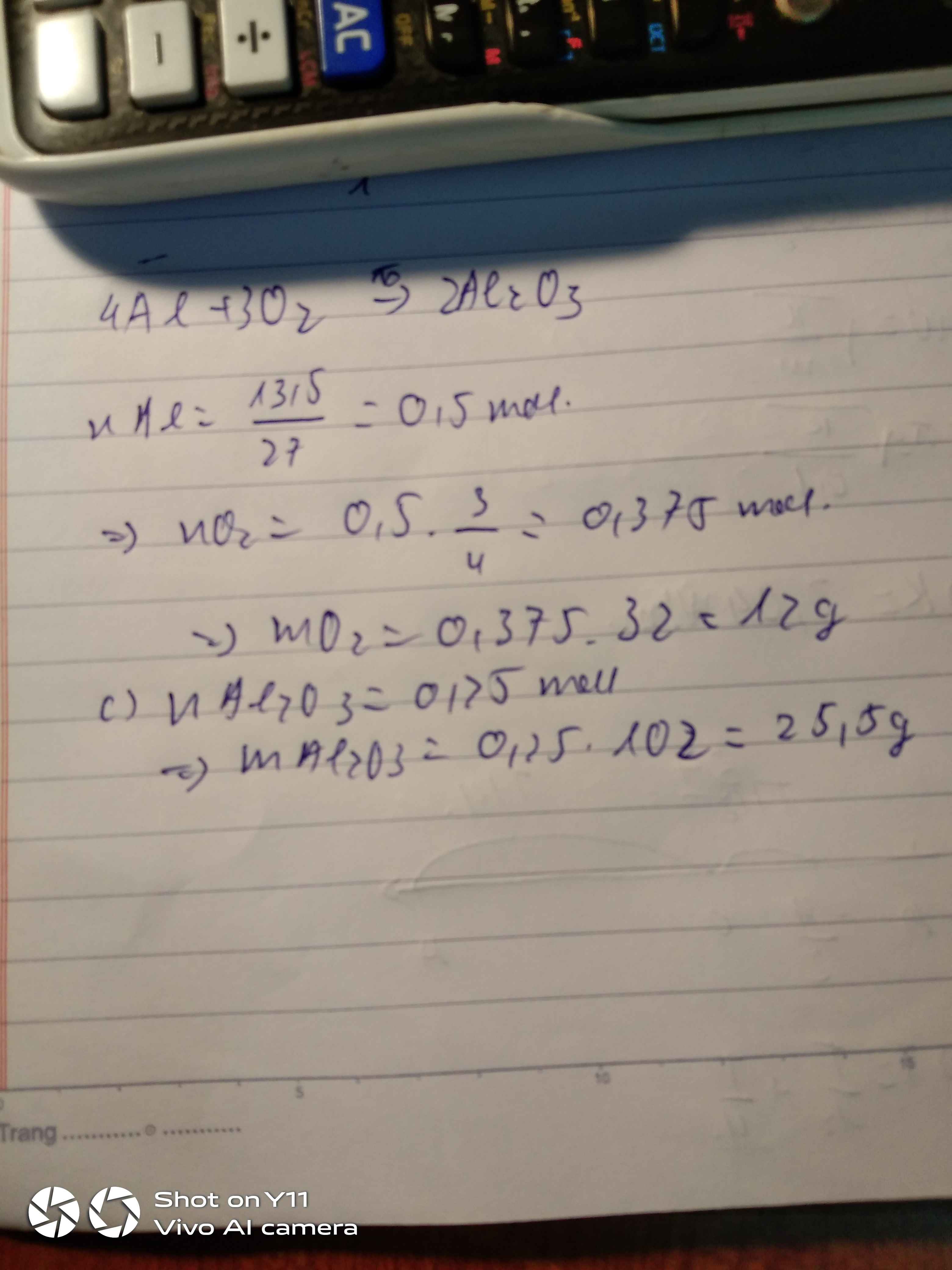a) $n_{Al} = \dfrac{13,5}{27} = 0,5(mol)$
$4Al +3 O_2 \xrightarrow{t^o} 2Al_2O_3$
Theo PTHH :
$n_{O_2} = \dfrac{3}{4}n_{Al} = 0,375(mol)$
$m_{O_2} = 0,375.32 = 12(gam)$
b)
$n_{Al_2O_3} = \dfrac{1}{2}n_{Al} = 0,25(mol)$
$m_{Al_2O_3} =0,25.102 = 25,5(gam)$
a,\(n_{Al}=\dfrac{13,5}{27}=0,5\left(mol\right)\)
PTHH: 4Al + 3O2 ---to→ 2Al2O3
Mol: 0,5 0,375 0,25
\(m_{O_2}=0,375.32=12\left(g\right)\)
b,\(m_{Al_2O_3}=0,25.102=25,5\left(g\right)\)