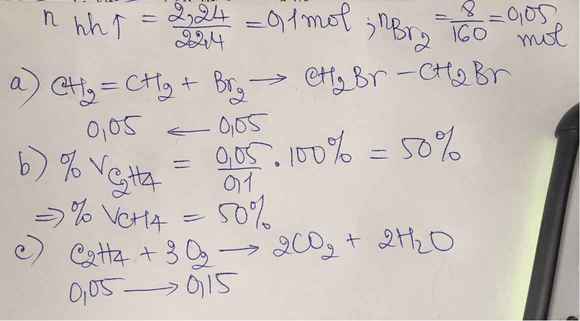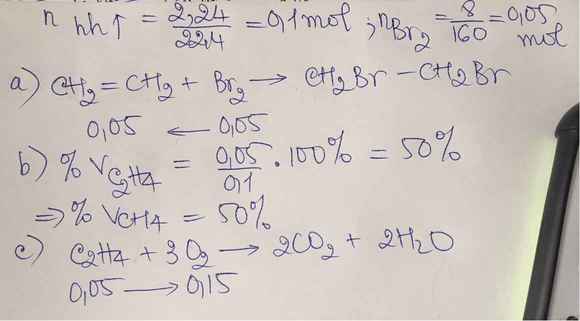\(n_{hh}=\dfrac{2,24}{22,4}=0,1mol\)
\(C_2H_4+Br_2\rightarrow C_2H_4Br_2\)
\(m_{Br_2}=8g\Rightarrow n_{Br_2}=\dfrac{8}{160}=0,05mol\Rightarrow n_{C_2H_4}=0,05mol\)
\(\Rightarrow n_{CH_4}=0,1-0,05=0,05mol\)
\(\%V_{CH_4}=\%V_{C_2H_4}=\dfrac{0,05}{0,1}\cdot100\%=50\%\)
c)Để đốt cháy:
\(CH_4+2O_2\underrightarrow{t^o}CO_2+2H_2O\)
\(C_2H_4+3O_2\underrightarrow{t^o}2CO_2+2H_2O\)
Từ hai pt trên:
\(\Rightarrow\Sigma n_{O_2}=2n_{CH_4}+3n_{C_2H_4}=2\cdot0,05+3\cdot0,05=0,25mol\)
\(\Rightarrow V_{O_2}=0,25\cdot22,4=5,6l\)
\(\Rightarrow V_{kk}=5,6\cdot5=28l\)