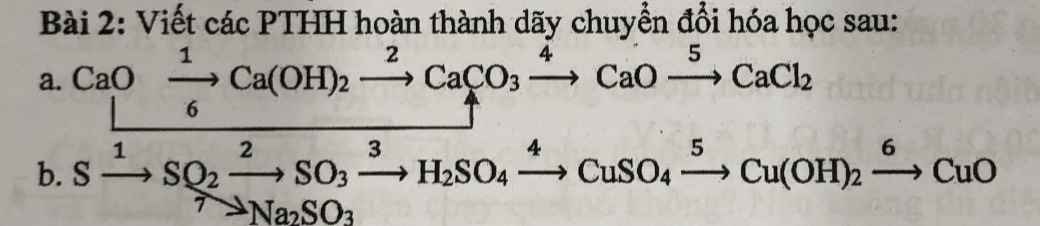
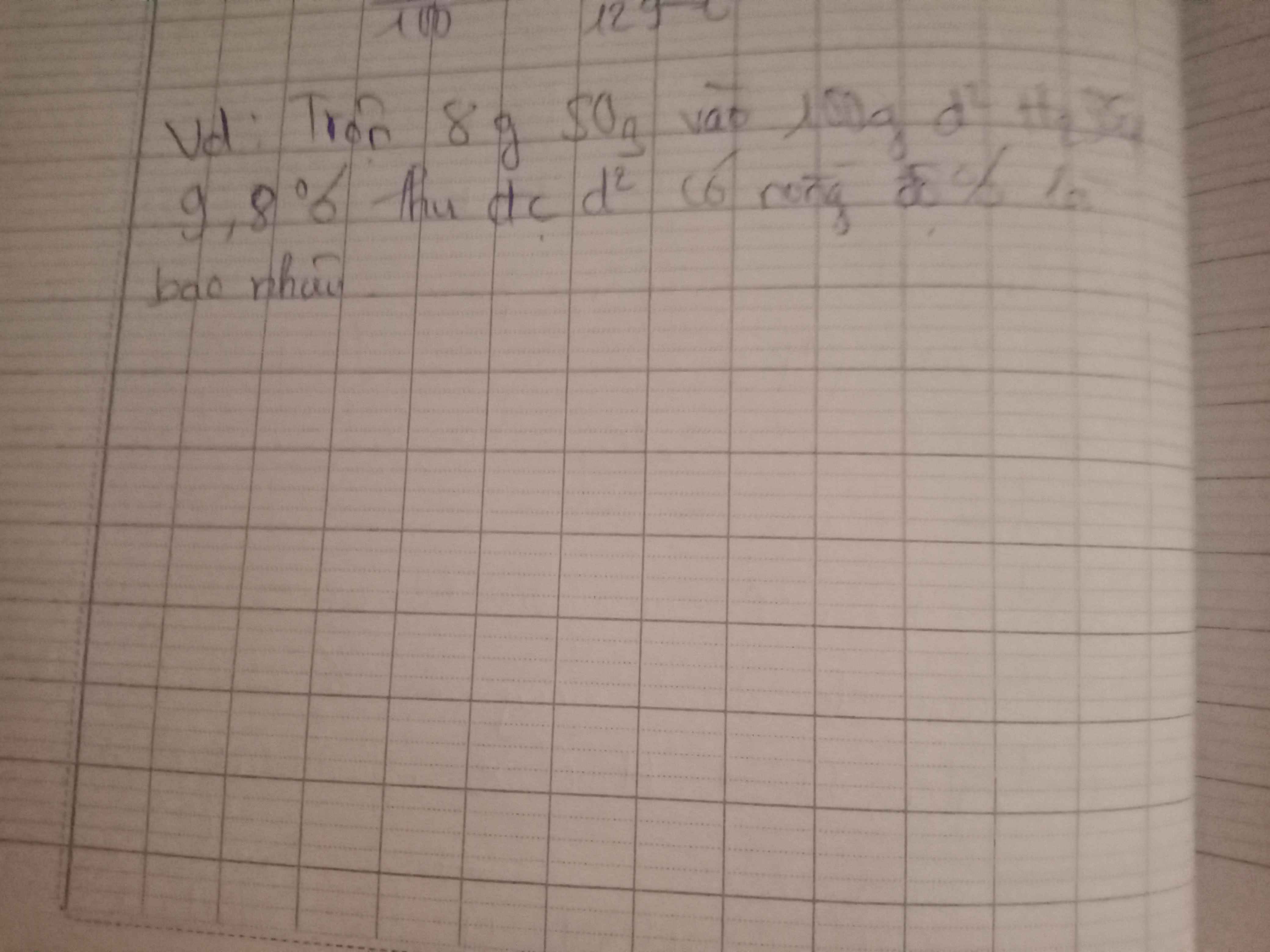
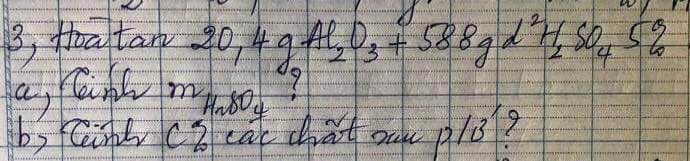a) $n_{H_2} = \dfrac{4,48}{22,4} = 0,2 (mol)$
PTHH:
Fe + 2HCl ---> FeCl2 + H2
0,2<-----------------------0,2
FeO + 2HCl ---> FeCl2 + H2O
b) \(\left\{{}\begin{matrix}\%m_{Fe}=\dfrac{0,2.56}{20}.100\%=56\%\\\%m_{FeO}=100\%-56\%=44\%\end{matrix}\right.\)