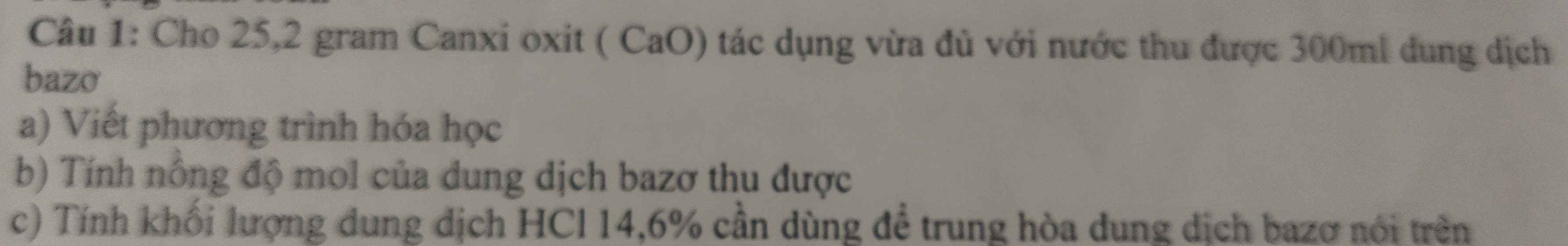Ta có: \(n_{H_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
\(a.PTHH:\)
\(Mg+2HCl--->MgCl_2+H_2\left(1\right)\)
\(CuO+2HCl--->CuCl_2+H_2O\left(2\right)\)
b. Theo PT(1): \(n_{Mg}=n_{H_2}=0,25\left(mol\right)\)
\(\Rightarrow m_{Mg}=0,25.24=6\left(g\right)\)
\(\Rightarrow m_{CuO}=24,25-6=18,25\left(g\right)\)
c. Ta có: \(n_{CuO}=\dfrac{18,25}{80}=\dfrac{73}{320}\left(mol\right)\)
\(\Rightarrow n_{hh}=\dfrac{73}{320}+0,25=0,478125\left(mol\right)\)
Theo PT(1,2): \(n_{HCl}=2.n_{hh}=2.0,478125=0,95625\left(mol\right)\)
Đổi 300ml = 0,3 lít
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,95625}{0,3}=3,1875M\)