PTHH: Mg + 2HCl ---> MgCl2 + H2
Ta có: \(n_{Mg}=\dfrac{48}{24}=2\left(mol\right)\)
Theo PT: \(n_{H_2}=n_{Mg}=2\left(mol\right)\)
=> \(V_{H_2}=2.22,4=44,8\left(lít\right)\)
Theo PT: \(n_{HCl}=2.n_{Mg}=2.2=4\left(mol\right)\)
=> \(m_{HCl}=4.36,5=146\left(g\right)\)
Đúng 0
Bình luận (0)






 GIÚP MIK VỚI Ạ!! MIK ĐANG CẦN GẤP
GIÚP MIK VỚI Ạ!! MIK ĐANG CẦN GẤP
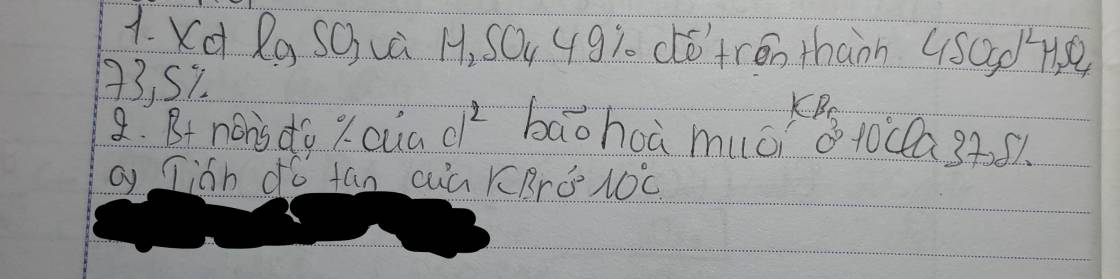 giải giúp mik vs ạ mik đang cần gấp!!!
giải giúp mik vs ạ mik đang cần gấp!!!







