nH2=4.48/22.4=0.2 mol
pt fe + 2hcl ---- fecl2 + h2 (1)
theopt(1) nfe=nfecl2=nh2=o,2 mol
=> mfe=0,2.56=11,2g
=>mfecl2=0,2.127=25,4g
\(a.\) \(Fe+2HCl\rightarrow FeCl_2+H_2\)
\(b.n_{H_2}=\dfrac{4,48}{22,4}=0,2mol\)
\(m_{Fe}=0,2.56=11,2g\)
\(c.m_{FeCl_2}=0,2.127=25,4g\)







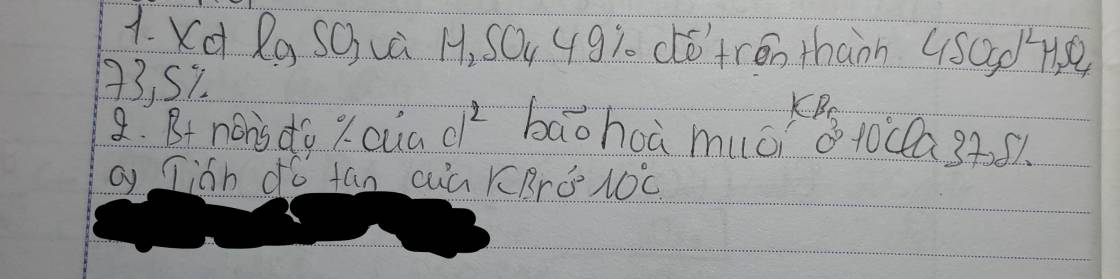 giải giúp mik vs ạ mik đang cần gấp!!!
giải giúp mik vs ạ mik đang cần gấp!!!








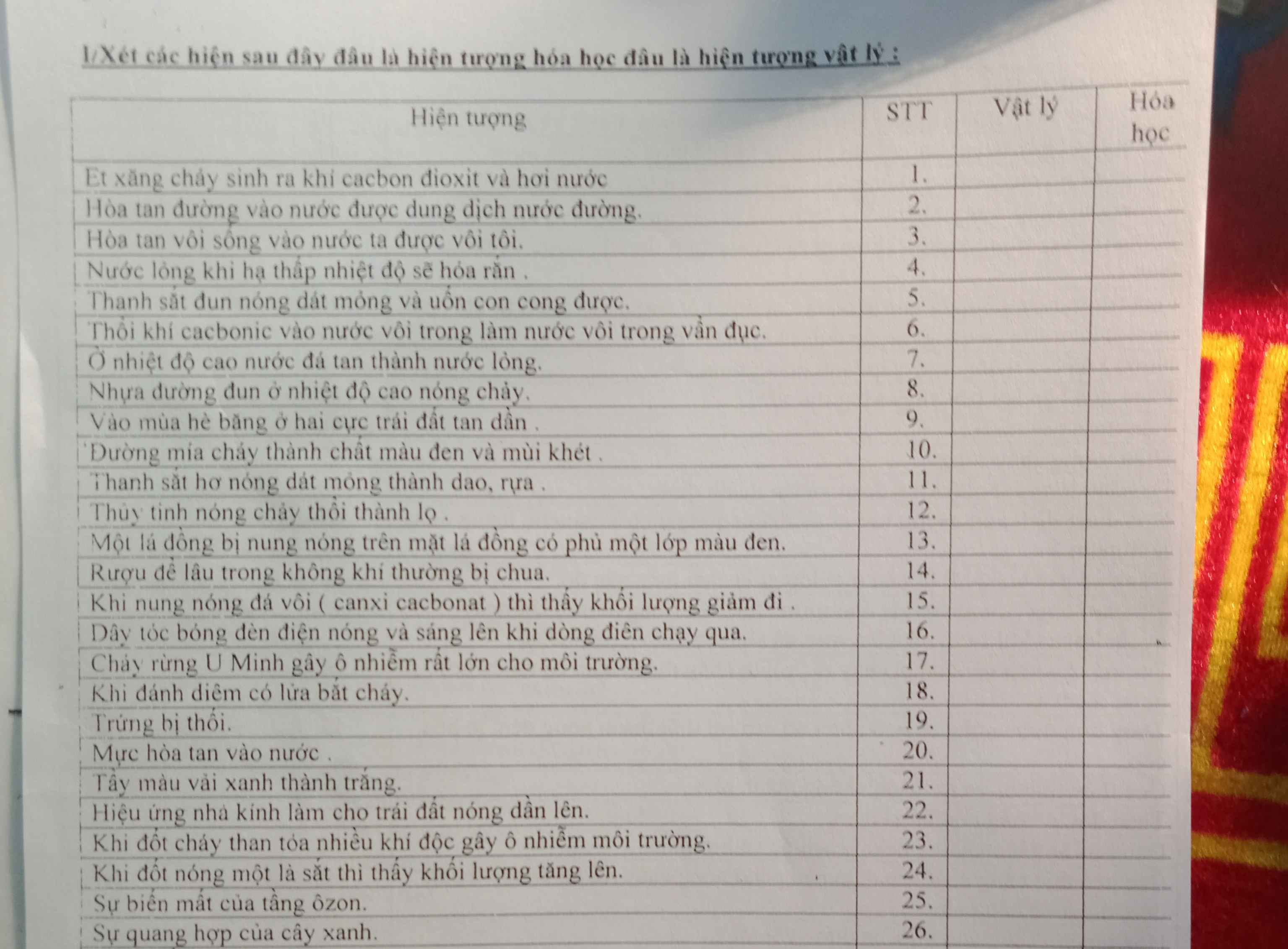

 GIÚP MIK VỚI Ạ!! MIK ĐANG CẦN GẤP
GIÚP MIK VỚI Ạ!! MIK ĐANG CẦN GẤP