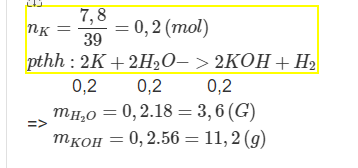\(n_K=\dfrac{7,8}{39}=0,2\left(mol\right)\\
pthh:2K+2H_2O->2KOH+H_2\)
0,2 0,2 0,2 0,1
=> \(V_{H_2}=0,1.22,4=2,24\left(L\right)\)
\(m_{H_2O}=0,2.18=3,6\left(g\right)\\
m_{KOH}=0,2.56=11,2\left(g\right)\)
a,2K+2H2O→2KOH+H2↑
b)VH2(đktc)=0,1.22,4=2,24(l)