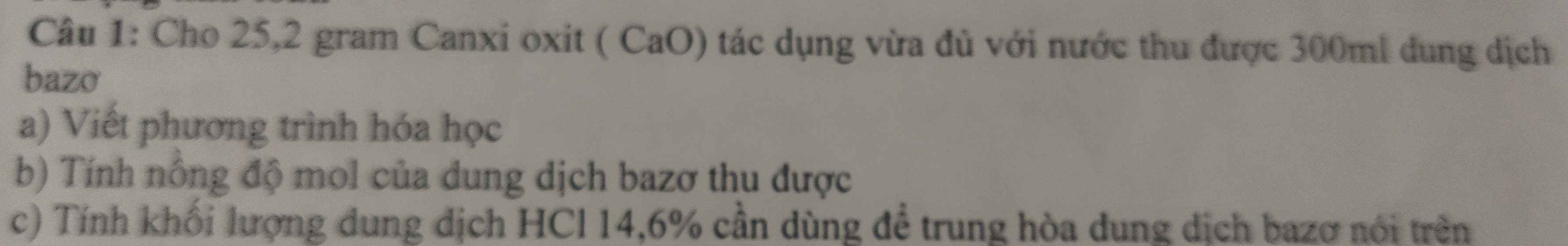a)
$Zn + 2HCl \to ZnCl_2 + H_2$
b)
$n_{H_2} = n_{Zn} = \dfrac{16,25}{65} = 0,25(mol)$
$V_{H_2} = 0,25.22,4 = 5,6(lít)$
c) $n_{HCl} = 2n_{Zn} = 0,5(mol) \Rightarrow V_{dd\ HCl} = \dfrac{0,5}{2,5} = 0,2(lít)$
d) $CuO + H_2 \xrightarrow{t^o} Cu + H_2O$
$n_{Cu} = n_{H_2} = 0,25(mol)$
$m_{Cu} = 0,25.64 = 16(gam)$
Đúng 2
Bình luận (0)