a, \(n_{\left(CH_3COO\right)_3Al}=\dfrac{20,4}{204}=0,1\left(mol\right)\)
6CH3COOH + 2Al → 2(CH3COO)3Al + 3H2
0,3 0,1
\(m_{CH_3COOH}=0,3.60=18\left(g\right)\Rightarrow C\%_{ddCH_3COOH}=\dfrac{18}{300}.100\%=6\%\)
b, \(m_{ddCH_3COOH\left(5\%\right)}=\dfrac{18.100\%}{5\%}=360\left(g\right)\)
\(m_{H_2O}=360-300=60\left(g\right)\)
c,
CH3COOH + KOH → CH3COOK + H2O
0,3 0,3
\(V_{ddKOH}=\dfrac{0,3}{1,5}=0,2\left(l\right)\)
\(n_{CH_3COOH}=\dfrac{300}{60}=5mol\)
\(n_{muối}=n_{\left(CH_3COO\right)_3Al}=\dfrac{20,4}{204}=0,1mol\)
\(6CH_3COOH+2Al\rightarrow2\left(CH_3COO\right)_3Al+3H_2\)
5 0 0,1 0
0,3 0,1 0,1 0,15
4,7 0,1 0 0,15
a)\(n_{CH_3COOH}=4,7\cdot60=282g\)
\(C\%=\dfrac{282}{300}\cdot100\%=94\%\)
c)Để trung hòa: \(n_{OH^-}=n_{COOH^-}=5mol\)
\(V_{dd}=\dfrac{5}{1,5}=3,33l\)








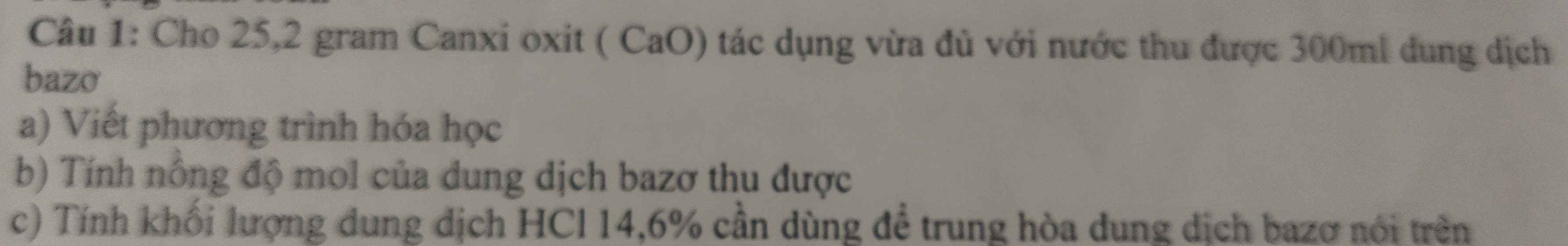











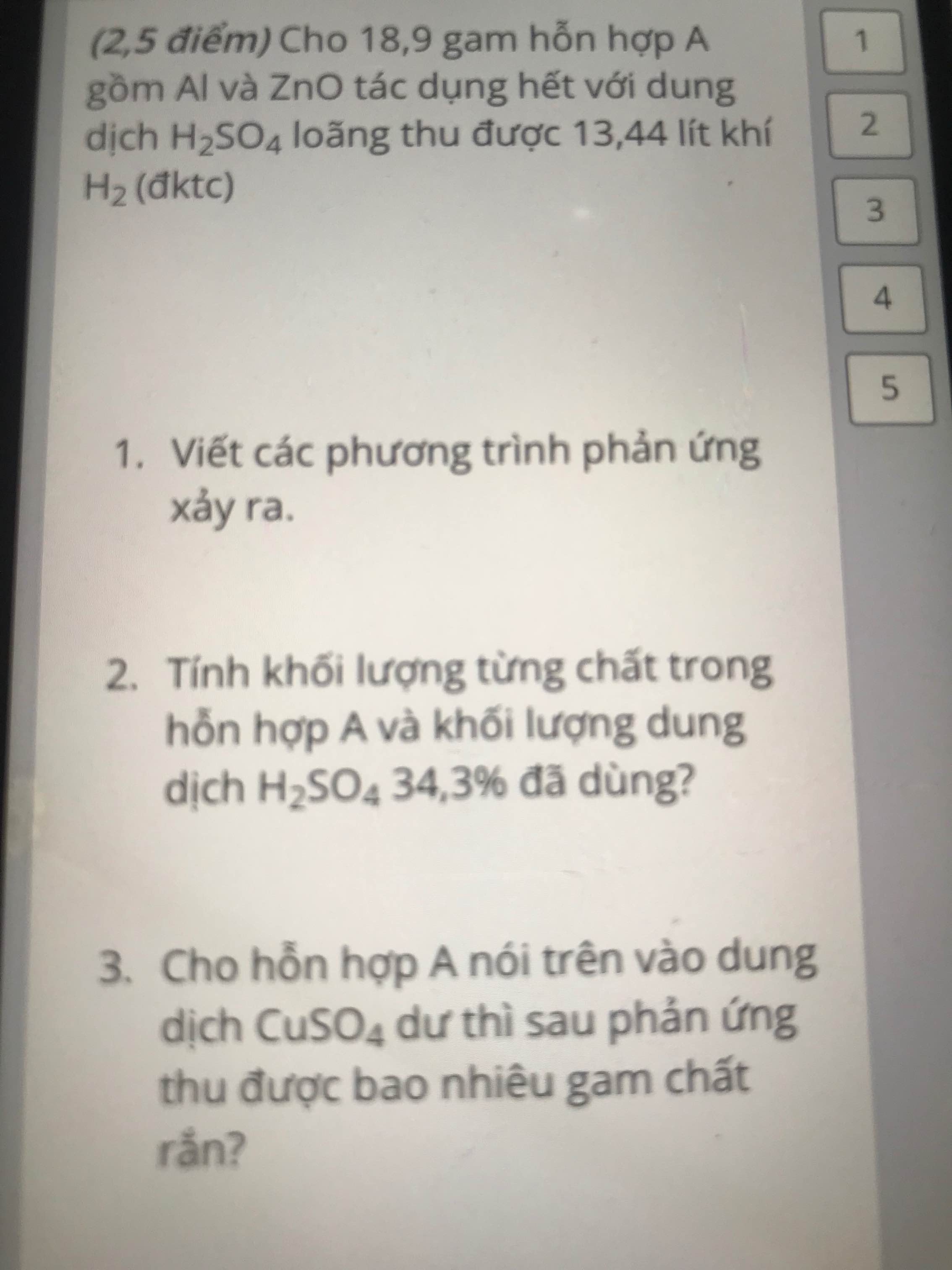
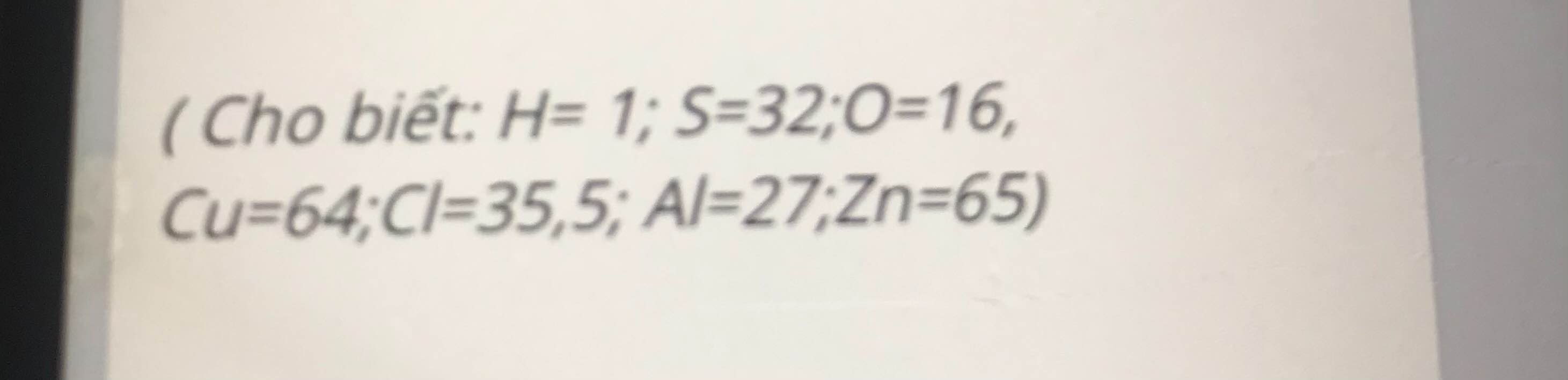

 giúp mình với mình cần gấp ạ
giúp mình với mình cần gấp ạ
