Bài 12:
\(n_{hh}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ M_X=\dfrac{12}{0,3}=40\left(\dfrac{g}{mol}\right)\\ Đặt:\%V_{O_2}=\%n_{O_2}=a\\ Vì:M_X=40\left(\dfrac{g}{mol}\right)\\ \Leftrightarrow\dfrac{32a+48\left(100\%-a\right)}{100\%}=40\\ \Leftrightarrow a=50\%\\ \Rightarrow\%V_{O_2}=\%V_{O_3}=50\%\)
Bài 31:
\(m_{NaCl}=\left(100\%-10,5\%\right).1=0,895\left(tấn\right)=895\left(kg\right)=895000\left(g\right)\\ m_{ddHCl}=1250.1,19=1487,5\left(g\right)\\ m_{HCl\left(p.ứ\right)}=37\%.1487,5=550,375\left(g\right)\\ NaCl+H_2SO_4\rightarrow\left(t^o\right)NaHSO_4+HCl\\ m_{HCl\left(LT\right)}=\dfrac{895000}{58,5}.36,5=558418,8034\left(g\right)\\ H=\dfrac{1487,5}{558418,8034}.100\approx0,26638\%\)
31:
\(m_{NaCl\left(bd\right)}=1000000-\dfrac{1000000.10,5}{100}=895000\left(g\right)\)
\(m_{ddHCl}=1250.10^3.1,19=1487500\left(g\right)\)
=> \(m_{HCl}=\dfrac{1487500.37}{100}=550375\left(g\right)\)
=> \(n_{HCl}=\dfrac{550375}{36,5}\left(mol\right)\)
=> \(n_{NaCl\left(pư\right)}=\dfrac{550375}{36,5}\left(mol\right)\)
=> \(H\%=\dfrac{\dfrac{550375}{36,5}.58,5}{895000}.100\%=98,56\%\)










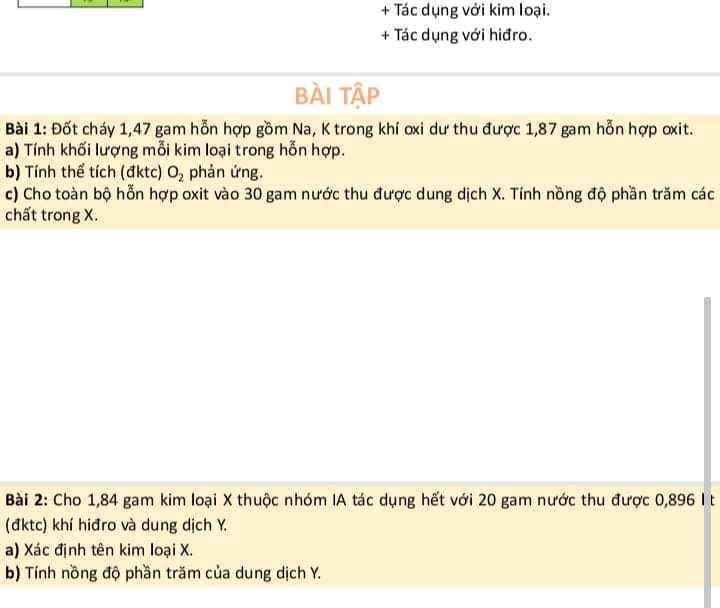







 giúp mình với ạ ;-; . mình đang cần gấp lắm
giúp mình với ạ ;-; . mình đang cần gấp lắm 






