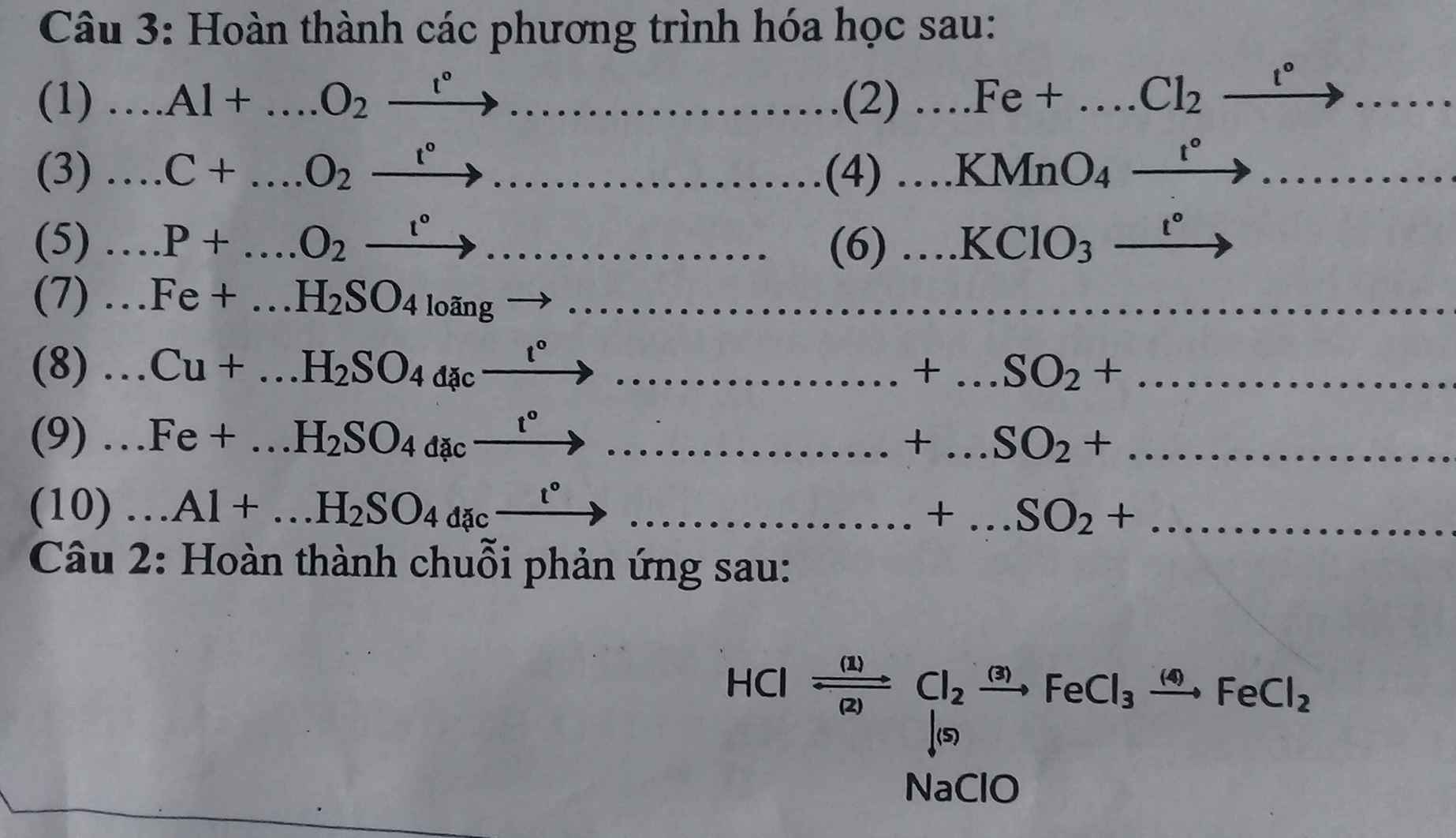
\(\%^{35}Cl=100\%-24,23\%=75,77\%\)
\(\overline{Cl}=\dfrac{37.24,23+35.75,77}{100}=35,4846\)
\(\%m_{^{37}Cl}=\dfrac{37.0,7577}{1+35,4846+16.4}.100\%\approx26,392\%\)
Sửa lại (bỏ phần giả sử về sau)
\(1\left(mol\right)HClO_4\rightarrow1\left(mol\right)Cl\)
\(\Rightarrow n\left(^{37}Cl\right)\) trong \(1\left(mol\right)HClO_4\) là \(1.24,23\%=0,2423\left(mol\right)\)
\(m\left(^{37}Cl\right)\) trong \(1\left(mol\right)HClO_4\) là \(0,2423.37=8,9651\left(g\right)\)
\(\Rightarrow\%m\left(^{37}Cl\right)=\dfrac{8,9651}{100,485}.100\%=8,92\%\)