\(M_{Na_3PO_4}=23.3+31+16.4=164\left(\dfrac{g}{mol}\right)\)
\(\%Na=\dfrac{23.3}{164}.100\%=42\%\)
\(\%P=\dfrac{31}{164}.100\%=18,9\%\)
\(\%O=100\%-42\%-18,9\%=39,1\%\)
Đúng 0
Bình luận (0)

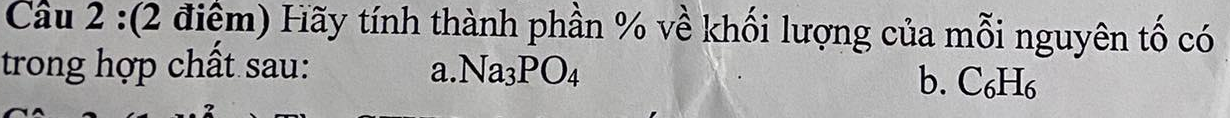
















 giúp mình câu 2,3 với ạ!!!
giúp mình câu 2,3 với ạ!!!
