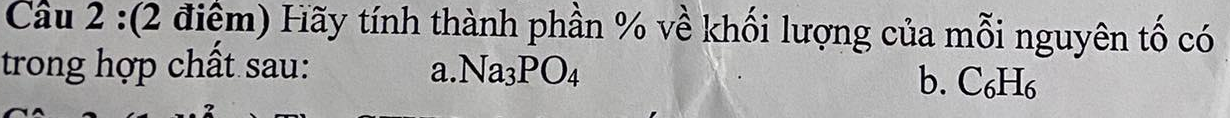Câu 5:
PTHH: \(2Al+6HCl\rightarrow2AlCl_3+3H_2\uparrow\)
a_____3a______a______ \(\dfrac{3}{2}a\) (mol)
\(Fe+2HCl\rightarrow FeCl_2+H_2\uparrow\)
b_____2b_____b______b (mol)
a) Ta lập được hệ phương trình: \(\left\{{}\begin{matrix}27a+56b=5,5\\\dfrac{3}{2}a+b=\dfrac{4,48}{22,4}=0,2\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,1\\b=0,05\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Al}=\dfrac{0,1\cdot27}{5,5}\cdot100\%\approx49,1\%\\\%m_{Fe}=50,9\%\end{matrix}\right.\)
b) Theo các PTHH: \(n_{HCl}=3n_{Al}+2n_{Fe}=0,4\left(mol\right)\) \(\Rightarrow V_{ddHCl}=\dfrac{0,4}{0,5}=0,8\left(l\right)\)
c) Theo PTHH: \(\left\{{}\begin{matrix}n_{AlCl_3}=0,1\left(mol\right)\\n_{FeCl_2}=0,05\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}C_{M_{AlCl_3}}=\dfrac{0,1}{0,8}=0,125\left(M\right)\\C_{M_{FeCl_2}}=\dfrac{0,05}{0,8}=0,0625\left(M\right)\end{matrix}\right.\)
Câu 6:
a+b) Ta có: \(\left\{{}\begin{matrix}\Sigma n_{H_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\n_{Zn}=\dfrac{9,75}{65}=0,15\left(mol\right)\end{matrix}\right.\)
PTHH: \(Zn+H_2SO_4\rightarrow ZnSO_4+H_2\uparrow\)
0,15___0,15_____0,15___0,15 (mol)
\(Fe+H_2SO_4\rightarrow FeSO_4+H_2\uparrow\)
0,25___0,25____0,25____0,25 (mol)
\(\Rightarrow m_{Fe}=0,25\cdot56=14\left(g\right)\)
c) PTHH: \(CuO+H_2\xrightarrow[]{t^o}Cu+H_2O\)
Ta có: \(\left\{{}\begin{matrix}n_{H_2}=0,4\left(mol\right)\\n_{CuO}=\dfrac{24}{80}=0,3\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) H2 còn dư, CuO p/ứ hết
\(\Rightarrow n_{Cu}=0,3\left(mol\right)\) \(\Rightarrow m_{Cu}=0,3\cdot64=19,2\left(g\right)\)