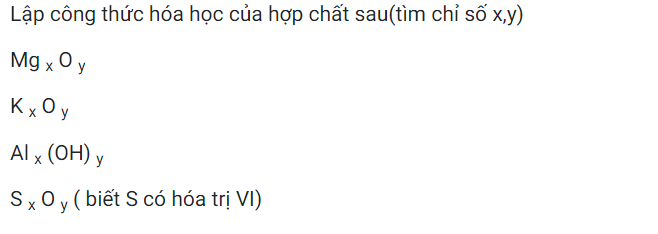
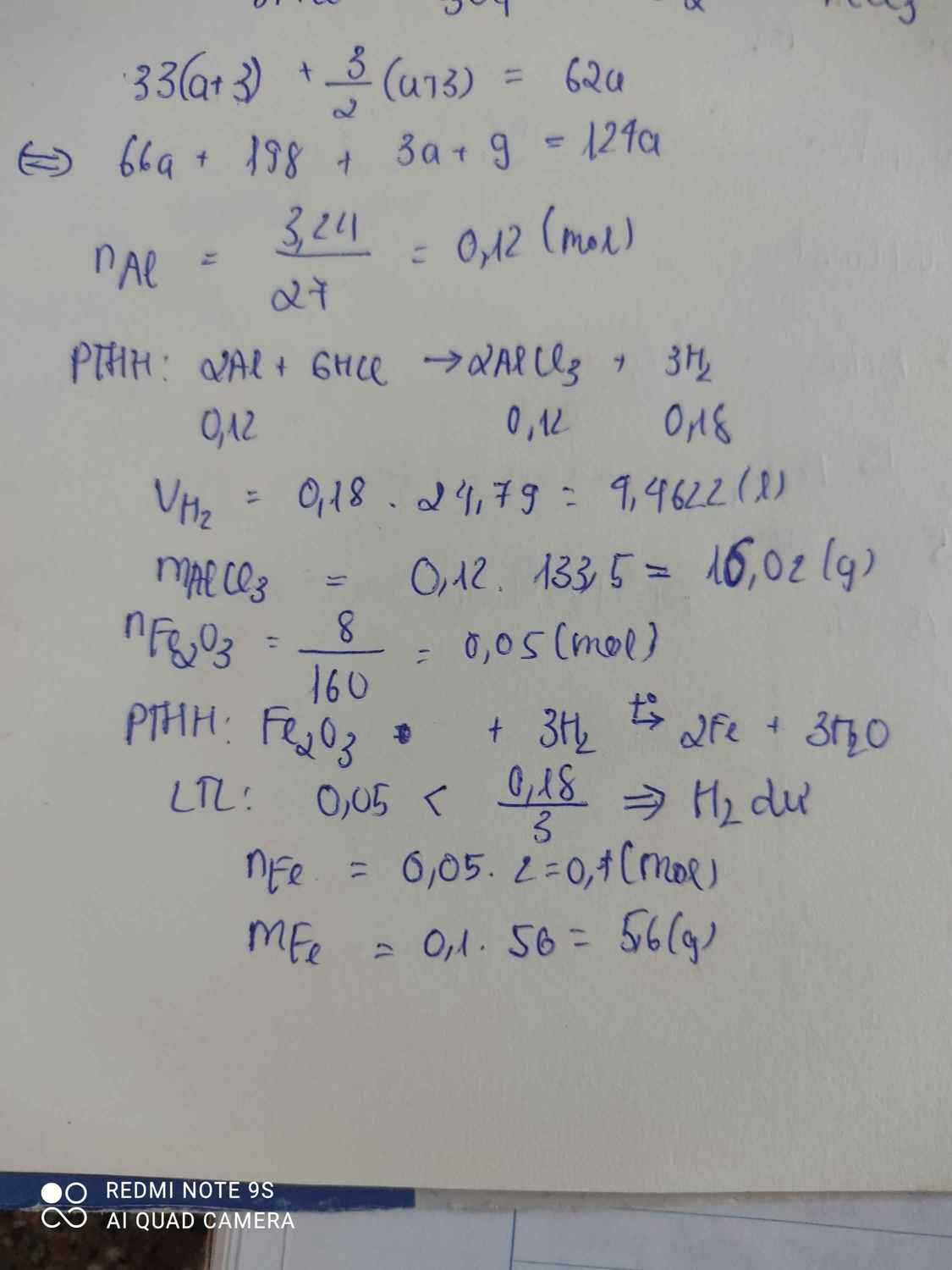a.b.\(n_{Al}=\dfrac{m}{M}=\dfrac{3,24}{27}=0,12mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,12 0,12 0,18 ( mol )
\(V_{H_2}=n.24,79=0,18.24,79=4,4622l\)
\(m_{AlCl_3}=n.M=0,12.133,5=16,02g\)
c.\(n_{Fe_2O_3}=\dfrac{m}{M}=\dfrac{8}{160}=0,05mol\)
\(Fe_2O_3+3H_2\rightarrow\left(t^o\right)2Fe+3H_2O\)
0,05 < 0,18 ( mol )
0,05 0,1 ( mol )
\(m_{Fe}=n.M=0,1.56=5,6g\)
nAl= 3,24:27=0,12(mol)
PTHH: 2Al + 6HCl ----> 2AlCl3+ 3H2(1)
theo pt (1), nH2 =3/2 nAl= 0,18 (mol)
=> VH2(ĐKC)= 0,18.24,79 =4,462(L)
Theo pt (1),nAlCl3 = nAl = 0,12 (mol)
=> mAlCl3 = 0,12 : 133,5 =16,02(g) / c) nFe2O3 = 8:160 =0,05 (mol)
pthh 3H2+ Fe2O3----> 2Fe + 3H2O(2)
theo pt (2) , nFe = 1/2nFe2O3 = 0,025(mol)
=> mFe = 0,025 . 56=1,4 (g)