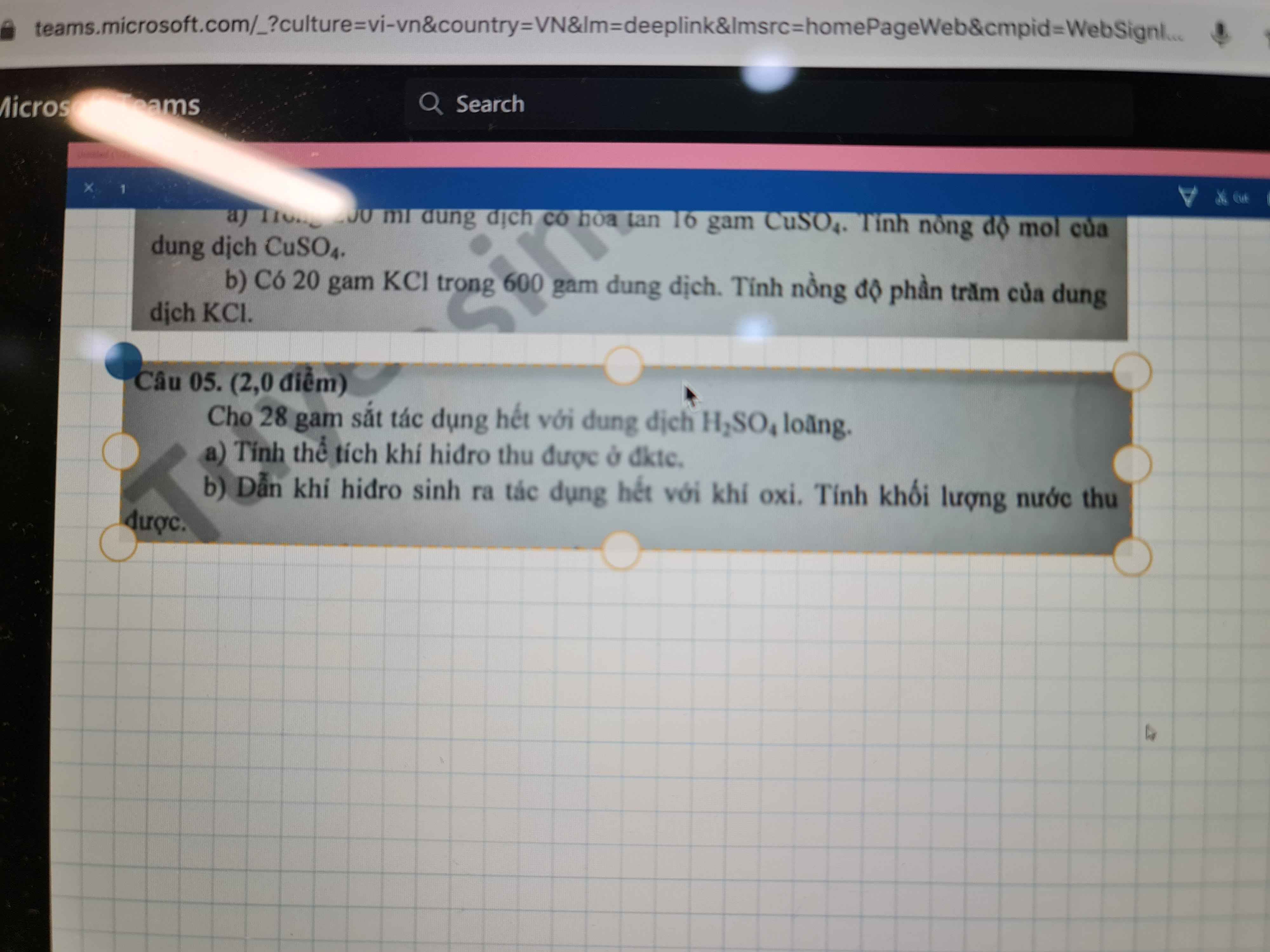
\(a,PTHH:Zn+2HCl\to ZnCl_2+H_2\\ b,n_{H_2}=\dfrac{2,24}{22,4}=0,1(mol)\\ \Rightarrow n_{Zn}=n_{ZnCl_2}=n_{H_2}=0,1(mol)\\ c,m_{Zn}=0,1.65=6,5(g)\\ m_{ZnCl_2}=0,1.136=13,6(g)\)
Đúng 1
Bình luận (0)










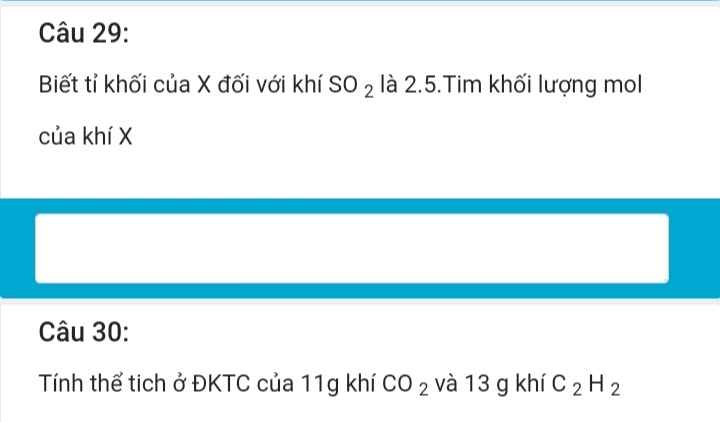



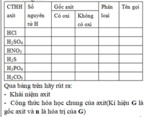



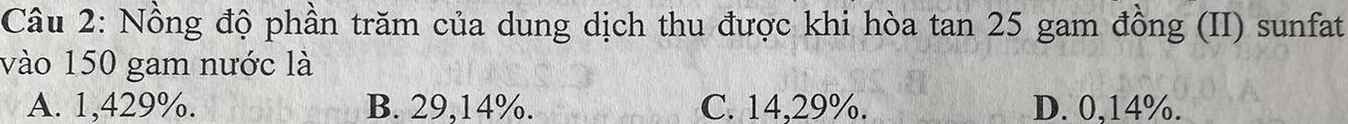 giải chi tiết giúp mình với ạ!!!!!!!!!
giải chi tiết giúp mình với ạ!!!!!!!!!