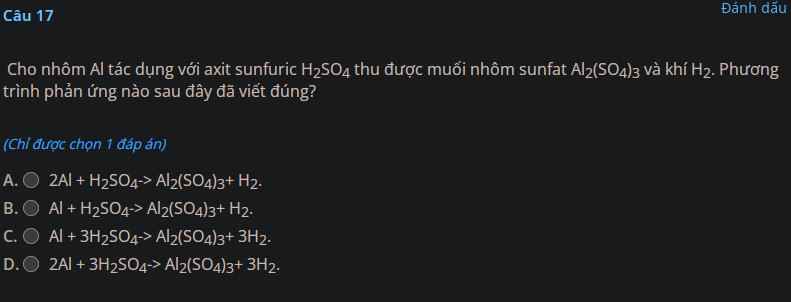
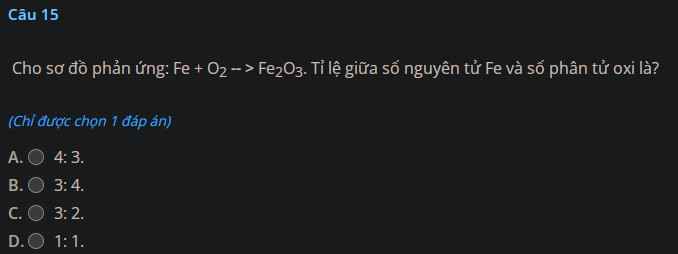
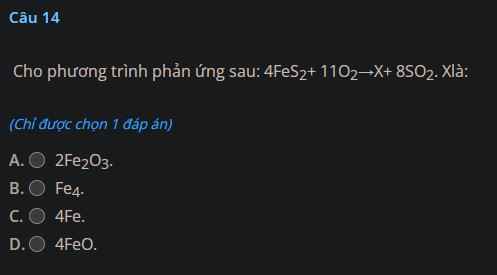
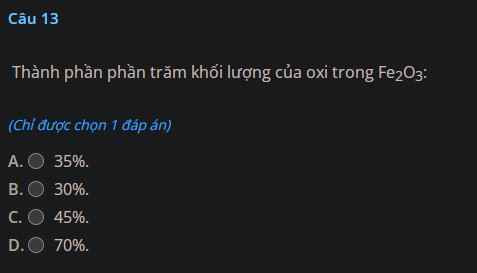
a) Zn + 2HCl $\to$ ZnCl2 + H2
b)
n H2 = n Zn = 9,75/65 = 0,15(mol)
V H2 = 0,15.22,4 = 3,36(lít)
n HCl = 2n H2 = 0,3 mol
=> m HCl = 0,3.36,5 = 10,95 gam
c) n CuO = 24/80 = 0,3(mol)
CuO + H2 $\xrightarrow{t^o}$ Cu + H2O
n CuO = 0,3 > n H2 = 0,15 => CuO dư
n Cu = n H2 = 0,15 mol
=> m Cu = 0,15.64 = 9,6 gam
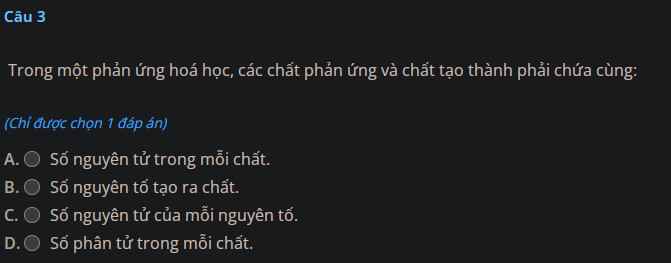
Câu 3 :
\(n_{Zn}=\dfrac{9.75}{65}=0.15\left(mol\right)\)
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)
\(0.15.......0.3..................0.15\)
\(V_{H_2}=0.15\cdot22.4=3.36\left(l\right)\)
\(m_{HCl}=0.3\cdot36.5=10.95\left(g\right)\)
\(n_{CuO}=\dfrac{24}{80}=0.3\left(mol\right)\)
\(CuO+H_2\underrightarrow{^{t^0}}Cu+H_2O\)
\(1..........1\)
\(0.3........0.15\)
\(LTL:\dfrac{0.3}{1}>\dfrac{0.15}{1}\Rightarrow CuOdư\)
\(n_{Cu}=n_{H_2}=0.15\left(mol\right)\)
\(m_{Cu}=0.15\cdot64=9.6\left(g\right)\)







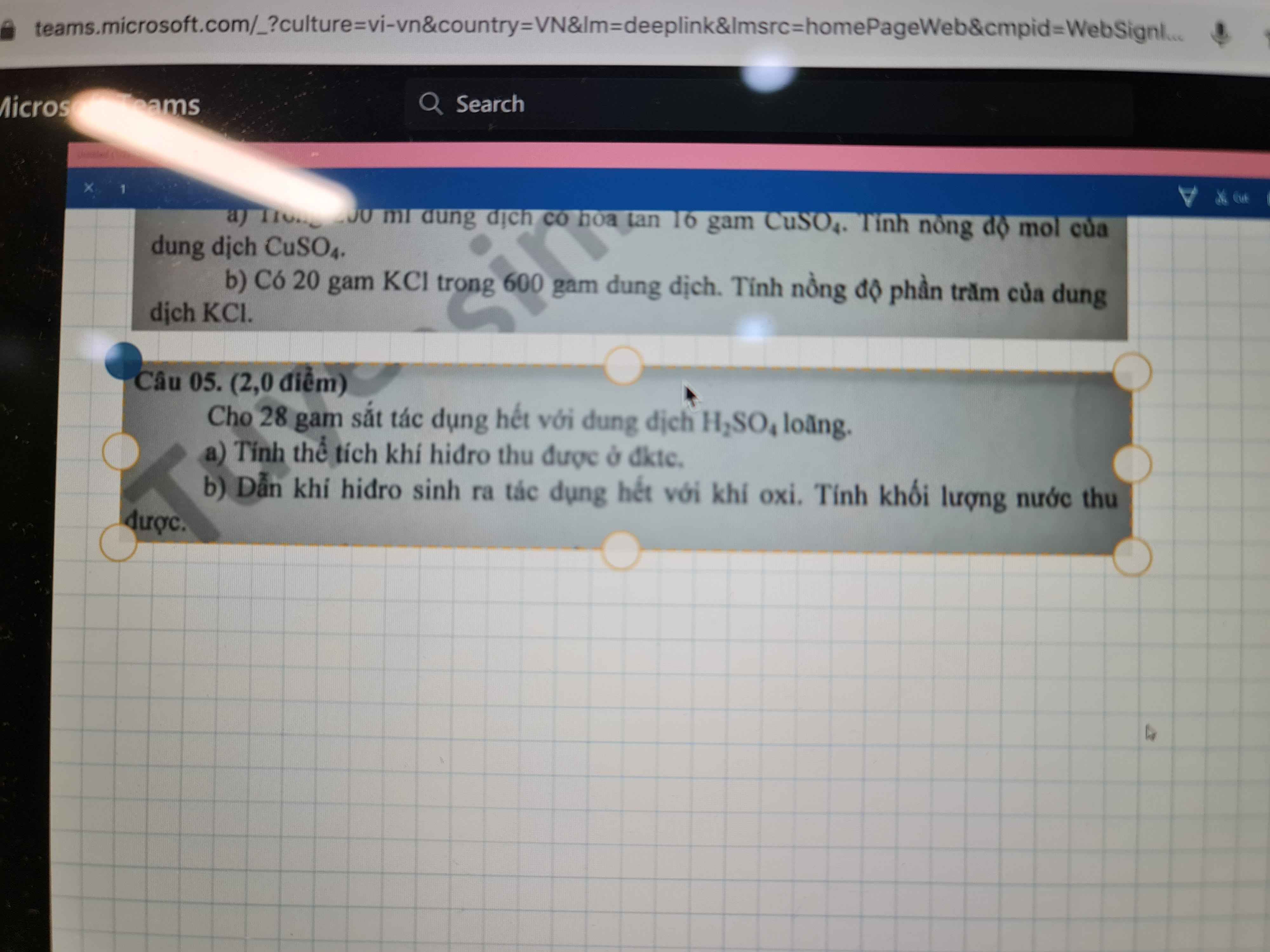
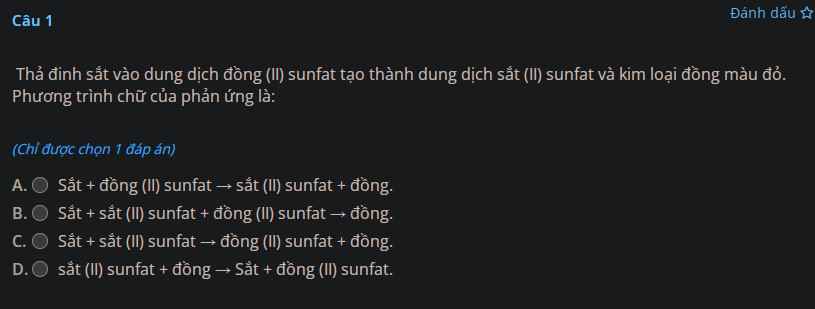
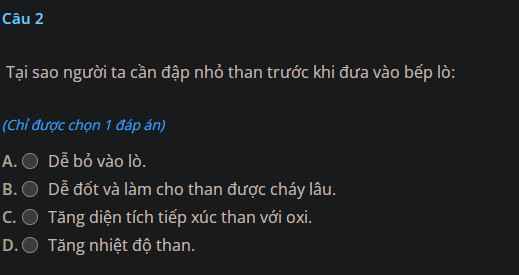
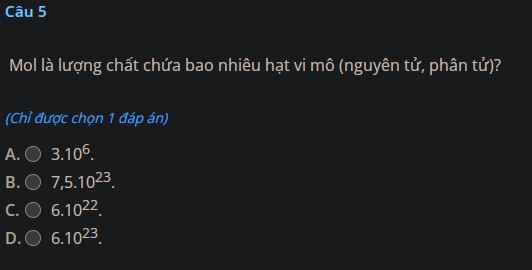
 Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )