\(n_{Ba\left(OH\right)_2}=\dfrac{150.10\%}{171}=\dfrac{5}{57}\left(mol\right)\)
PTHH: `Ba(OH)_2 + 2HCl -> BaCl_2 + 2H_2O`
\(\dfrac{5}{57}\)--------->\(\dfrac{10}{57}\)
\(\Rightarrow m_{HCl}=\dfrac{\dfrac{10}{57}.36,5}{15\%}=\dfrac{7300}{171}\left(g\right)\)
Đúng 1
Bình luận (0)






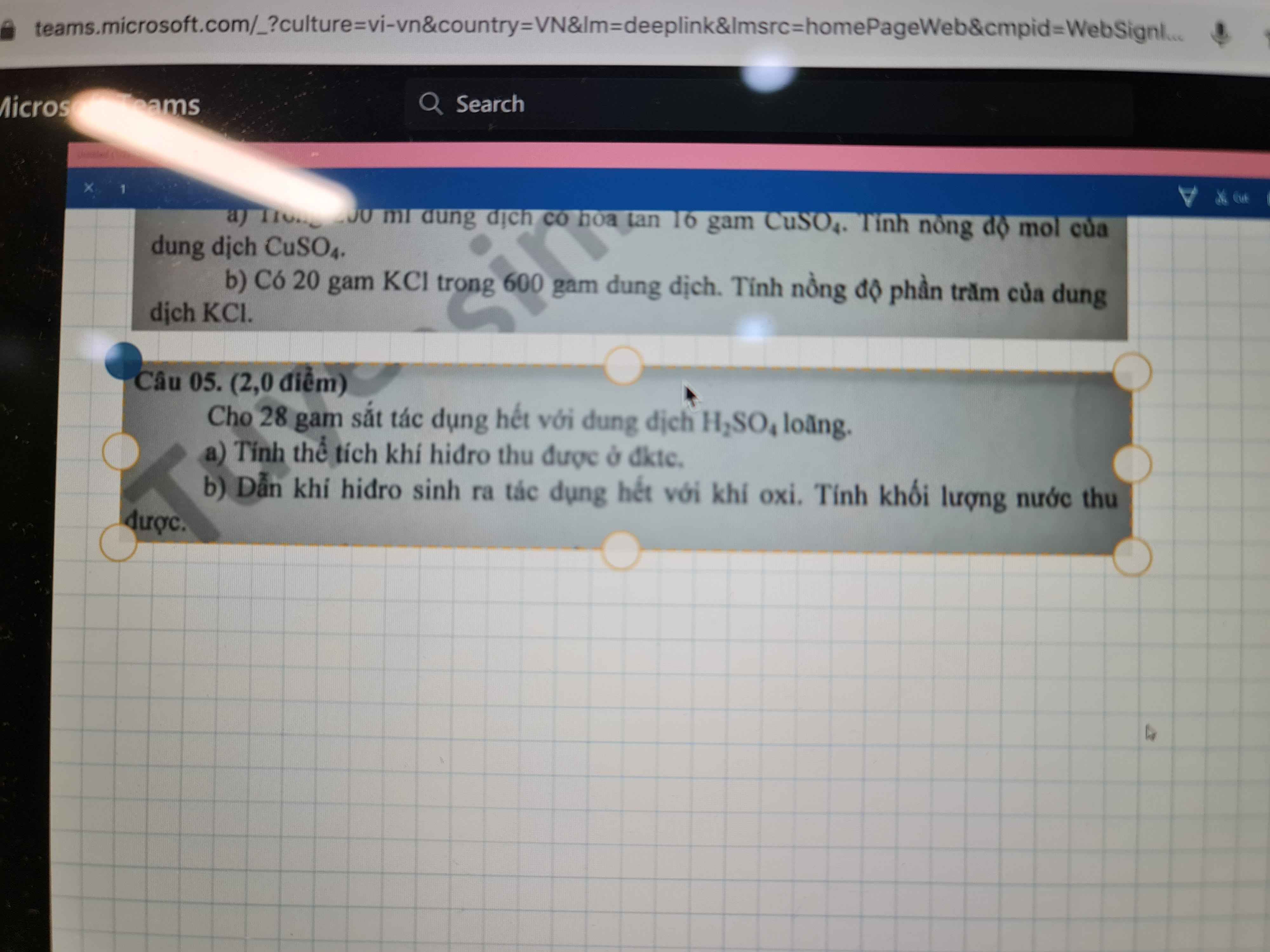
 Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )
Giúp mình giải các câu hỏi trên trừ 2 câu khoanh tròn vì mình đã làm r giúp mik với :3 cảm ơn nhiều ( lưu ý : giải chi tiết vì mình đang bắt đầu học )


 Giải giúp mình câu 10 ik giải chi tiết nha
Giải giúp mình câu 10 ik giải chi tiết nha