a)
(1) `Mg + H_2SO_4 -> MgSO_4 + H_2`
(2) \(MgSO_4+BaCl_2\rightarrow BaSO_4\downarrow+MgCl_2\)
(3) \(MgCl_2+Ca\left(OH\right)_2\rightarrow Mg\left(OH\right)_2\downarrow+CaCl_2\)
(4) \(Mg\left(OH\right)_2\xrightarrow[]{t^o}MgO+H_2O\)
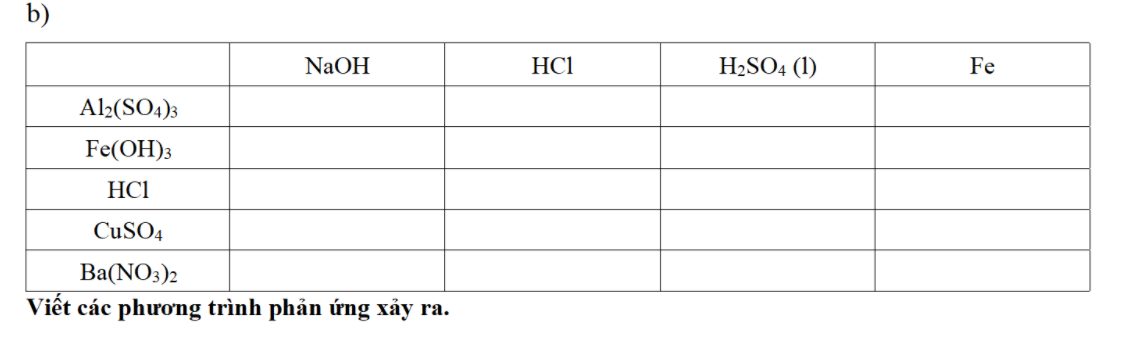
b)
(1) `Zn + H_2SO_4 -> ZnSO_4 + H_2`
(2) \(ZnSO_4+BaCl_2\rightarrow BaSO_4\downarrow+ZnCl_2\)
(3) \(ZnCl_2+2KOH\rightarrow Zn\left(OH\right)_2\downarrow+2KCl\)
(4) \(Zn\left(OH\right)_2\xrightarrow[]{t^o}ZnO+H_2O\)
c)
(1) \(Al_2\left(SO_4\right)_3+3Ba\left(OH\right)_2\rightarrow Al\left(OH\right)_3\downarrow+3BaSO_4\downarrow\)
(2) \(2Al\left(OH\right)_3\xrightarrow[]{t^o}Al_2O_3+3H_2O\)
(3) \(2Al_2O_3\xrightarrow[Na_3AlF_6]{đpnc}4Al+3O_2\)
(4) \(2Al+3S\xrightarrow[]{t^o}Al_2S_3\)
d)
(1) \(Fe\left(NO_3\right)_3+3KOH\rightarrow Fe\left(OH\right)_3\downarrow+3KNO_3\)
(2) \(Fe\left(OH\right)_3+3HCl\rightarrow FeCl_3+3H_2O\)
(3) \(Al+FeCl_3\rightarrow AlCl_3+Fe\downarrow\)
(4) \(3Fe+2O_2\xrightarrow[]{t^o}Fe_3O_4\)