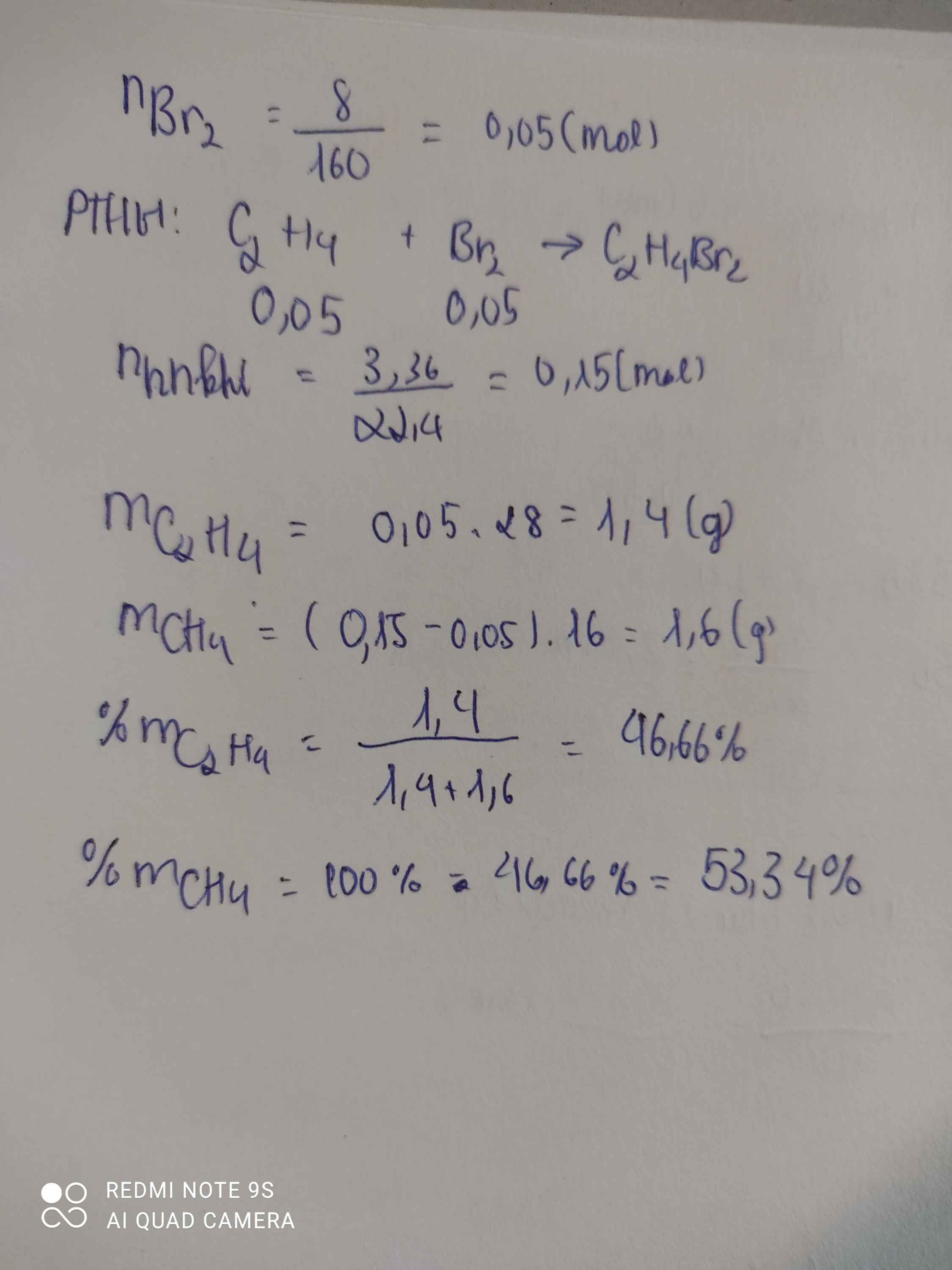a)
\(n_{Br_2}=\dfrac{8}{160}=0,05\left(mol\right)\)
PTHH: C2H4 + Br2 --> C2H4Br2
0,05<-0,05
=> \(n_{CH_4}=\dfrac{3,36}{22,4}-0,05=0,1\left(mol\right)\)
\(\%m_{CH_4}=\dfrac{0,1.16}{0,1.16+0,05.28}.100\%=53,33\%\)
\(\%m_{C_2H_4}=\dfrac{0,05.28}{0,1.16+0,05.28}.100\%=46,67\%\)
b)
PTHH: CH4 + 2O2 --to--> CO2 + 2H2O
0,1-->0,2
C2H4 + 3O2 --to--> 2CO2 + 2H2O
0,05--->0,15
=> \(V_{O_2}=\left(0,2+0,15\right).22,4=7,84\left(l\right)\)