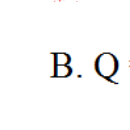a) $2Al + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2$
b)
Bảo toàn khối lượng :
$m_{Al} + m_{H_2SO_4} = m_{Al_2(SO_4)_3} + m_{H_2}$
Suy ra :
$m_{H_2SO_4} = 171 + 3 - 27 = 147(gam)$
a) PTHH:2Al + 3 H2SO4 -> Al2(SO4)3 + 3H2
b) Theo ĐLBTKL:
\(m_{Al}+m_{H_2SO_4}=m_{Al_2\left(SO_4\right)_3}+m_{H_2}\\ \Leftrightarrow27+m_{H_2SO_4}=171+3\\ \Leftrightarrow m_{H_2SO_4}=147\left(g\right)\)