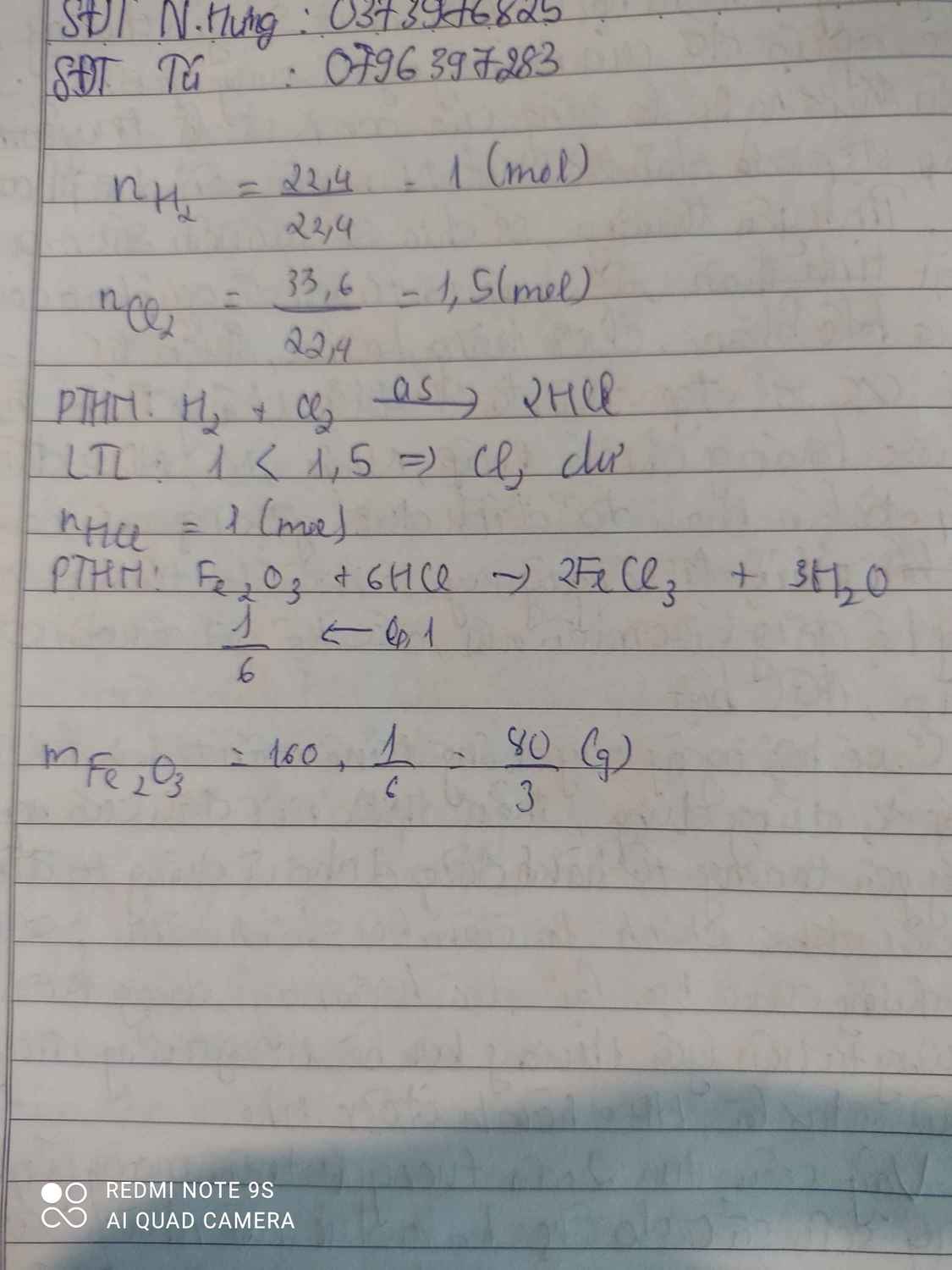\(n_{H_2}=\dfrac{22,4}{22,4}=1\left(mol\right);n_{Cl_2}=\dfrac{33,6}{22,4}=1,5\left(mol\right)\\ PTHH:H_2+Cl_2\rightarrow\left(as\right)2HCl\\ Vì:\dfrac{1}{1}>\dfrac{1,5}{1}\Rightarrow Cl_2dư\\ n_{HCl\left(thu.được\right)}=75\%.n_{H_2}=75\%.1=0,75\left(mol\right)\\ Fe_2O_3+6HCl\rightarrow2FeCl_3+3H_2O\\ n_{Fe_2O_3}=\dfrac{n_{HCl}}{6}=\dfrac{0,75}{6}=0,125\left(mol\right)\\ \Rightarrow m_{Fe_2O_3}=160.0,125=20\left(g\right)\)
Đúng 3
Bình luận (9)
Các câu hỏi tương tự
Cho 10,00 lít H2 và 6,72 lít khí Clo (đktc) tác dụng với nhau rồi hòa tan sản phẩm vào 385,40 gam nước thu được dung dịch A. Lấy 50 gam dung dịch A cho tác dụng với AgNO3 dư thu được 7,175 gam kết tủa. Tính hiệu suất phản ứng giữa H2 và Cl2
Cho 2 lít (đktc) H2 tác dụng với 1,344 lít Cl2 (đktc) rồi hòa tan sản phẩm vào nước để được 40 gam dụng dịch A. Lấy 10 gam A tác dụng với dung dịch AgNO3 dư thu được 3,444 gam kết tủa. Tính hiệu suất phản ứng giữa H2 và Cl2 (giả sử Cl2 và H2 không tan trong nước)? A. 20% B. 80% C. 40% D. 50%
Đọc tiếp
Cho 2 lít (đktc) H2 tác dụng với 1,344 lít Cl2 (đktc) rồi hòa tan sản phẩm vào nước để được 40 gam dụng dịch A. Lấy 10 gam A tác dụng với dung dịch AgNO3 dư thu được 3,444 gam kết tủa. Tính hiệu suất phản ứng giữa H2 và Cl2 (giả sử Cl2 và H2 không tan trong nước)?
A. 20%
B. 80%
C. 40%
D. 50%
Cho 10,0 lít H2 và 6,72 lít Cl2 (đktc) tác dụng với nhau rồi hòa tan sản phẩm vào 358,4 gam nước ta thu được dung dịch A. Lấy 50,0 gam dung dịch A tác dụng với dung dịch AgNO3 dư thu được 7,175 gam kết tủa. Hiệu suất của phản ứng giữa H2 và Cl2 là: A. 32,4% B. 20,0% C. 44,8% D. 66,7%
Đọc tiếp
Cho 10,0 lít H2 và 6,72 lít Cl2 (đktc) tác dụng với nhau rồi hòa tan sản phẩm vào 358,4 gam nước ta thu được dung dịch A. Lấy 50,0 gam dung dịch A tác dụng với dung dịch AgNO3 dư thu được 7,175 gam kết tủa. Hiệu suất của phản ứng giữa H2 và Cl2 là:
A. 32,4%
B. 20,0%
C. 44,8%
D. 66,7%
Hòa tan hoàn toàn 57,6 gam hỗn hợp A gồm Fe3O4, Fe2O3, FeO, Fe trong dung dịch HCl thì cần dùng 360 gam dung dịch HCl 18,25% để tác dụng vừa đủ. Sau phản ứng thu được V lít H2 (đktc) và dung dịch B. Cho toàn bộ H2 sinh ra tác dụng hết với CuO dư ở điều kiện nhiệt độ cao, sau phản ứng thu được hỗn hợp rắn gồm Cu và CuO có khối lượng nhỏ hơn khối lượng CuO ban đầu là 3,2 gam.
a) Nếu cô cạn dung dịch B, ta thu được bao nhiêu gam muối khan?
b) Nếu hỗn hợp A ban đầu có tỉ lệ mol Fe2O3 : FeO 1 : 1....
Đọc tiếp
Hòa tan hoàn toàn 57,6 gam hỗn hợp A gồm Fe3O4, Fe2O3, FeO, Fe trong dung dịch HCl thì cần dùng 360 gam dung dịch HCl 18,25% để tác dụng vừa đủ. Sau phản ứng thu được V lít H2 (đktc) và dung dịch B. Cho toàn bộ H2 sinh ra tác dụng hết với CuO dư ở điều kiện nhiệt độ cao, sau phản ứng thu được hỗn hợp rắn gồm Cu và CuO có khối lượng nhỏ hơn khối lượng CuO ban đầu là 3,2 gam. a) Nếu cô cạn dung dịch B, ta thu được bao nhiêu gam muối khan? b) Nếu hỗn hợp A ban đầu có tỉ lệ mol Fe2O3 : FeO = 1 : 1. Tính nồng độ phần trăm các chất có trong dung dịch B. c) Hỗn hợp X cũng chứa Fe3O4, Fe2O3, FeO, Fe. Nếu dùng 100 gam X cho tác dụng với 2 lít dung dịch HCl 2M. Chứng minh rằng hỗn hợp X tan hết. Ghi rõ pt và giải rõ a b c giúp e vs ạ
. cho 1 lít khí H2 và 0,672 lít khí Cl2(dktc) tác dụng với nhau rồi hòa tan sản phẩm vào 38,54g nước ta thu được dung dịch A. Lấy dung dịch A trên cho tác dụng với dung dịch AgNO3 (lấy dư) thu được 7,175g kết tủa. Tính hiệu suất của phản ứng giữa H2 và Cl2
Cho 69,6 gam MnO2 tác dụng với HCl đặc, dư.(H%90%). Dẫn toàn bộ lượng khí sinh ra vào 500 ml dung dịch NaOH 4M thu được dung dịch A. Thể tích khí Cl2 thu được ở đktc sau phản ứng là: A. 17,92 B. 16,128 C. 19,9 D. 13,44
Đọc tiếp
Cho 69,6 gam MnO2 tác dụng với HCl đặc, dư.(H%=90%). Dẫn toàn bộ lượng khí sinh ra vào 500 ml dung dịch NaOH 4M thu được dung dịch A. Thể tích khí Cl2 thu được ở đktc sau phản ứng là:
A. 17,92
B. 16,128
C. 19,9
D. 13,44
Cho 7,5 gam hỗn hợp X gồm kim loại M (hóa trị không đổi) và Mg (tỉ lệ mol tương ứng 2 : 3) tác dụng với 3,36 lít Cl2, thu được hỗn hợp rắn Y. Hòa tan hết toàn bộ Y trong lượng dư dung dịch HCl, thu được 1,12 lít H2. Biết các phản ứng đều xảy ra hoàn toàn, các thể tích khí đều đo ở đktc. Kim loại M là Cho 7,5 gam hỗn hợp X gồm kim loại M (hóa trị không đổi) và Mg (tỉ lệ mol tương ứng 2 : 3) tác dụng với 3,36 lít Cl2, thu được hỗn hợp rắn Y. Hòa tan hết toàn bộ Y trong lượng dư dung dịch HCl, th...
Đọc tiếp
Cho 7,5 gam hỗn hợp X gồm kim loại M (hóa trị không đổi) và Mg (tỉ lệ mol tương ứng 2 : 3) tác dụng với 3,36 lít Cl2, thu được hỗn hợp rắn Y. Hòa tan hết toàn bộ Y trong lượng dư dung dịch HCl, thu được 1,12 lít H2. Biết các phản ứng đều xảy ra hoàn toàn, các thể tích khí đều đo ở đktc. Kim loại M là
Cho 7,5 gam hỗn hợp X gồm kim loại M (hóa trị không đổi) và Mg (tỉ lệ mol tương ứng 2 : 3) tác dụng với 3,36 lít Cl2, thu được hỗn hợp rắn Y. Hòa tan hết toàn bộ Y trong lượng dư dung dịch HCl, thu được 1,12 lít H2. Biết các phản ứng đều xảy ra hoàn toàn, các thể tích khí đều đo ở đktc. Kim loại M là
Cho 7,5 gam hỗn hợp X gồm kim loại M (hóa trị không đổi) và Mg (tỉ lệ mol tương ứng 2 : 3) tác dụng với 3,36 lít Cl2, thu được hỗn hợp rắn Y. Hòa tan hết toàn bộ Y trong lượng dư dung dịch HCl, thu được 1,12 lít H2. Biết các phản ứng đều xảy ra hoàn toàn, các thể tích khí đều đo ở đktc. Kim loại M là
A. Al.
B. Na
C. Ca.
D. K.
Cho 8,7 gam MnO2 tác dụng với dung dịch axit HCl đậm đặc sinh ra V lít khí Cl2 (đktc). Hiệu suất phản ứng là 85%. V có giá trị là:
A. 2 lít
B. 2,905 lít
C. 1,904 lít
D. 1,82 lít
Cho 8,7 gam MnO2 tác dụng với dung dịch axit HCl đậm đặc sinh ra V lít khí Cl2 (đktc). Hiệu suất phản ứng là 85%. V có giá trị là :
A. 2 lít
B. 2,905 lít
C. 1,904 lít
D. 1,82 lít