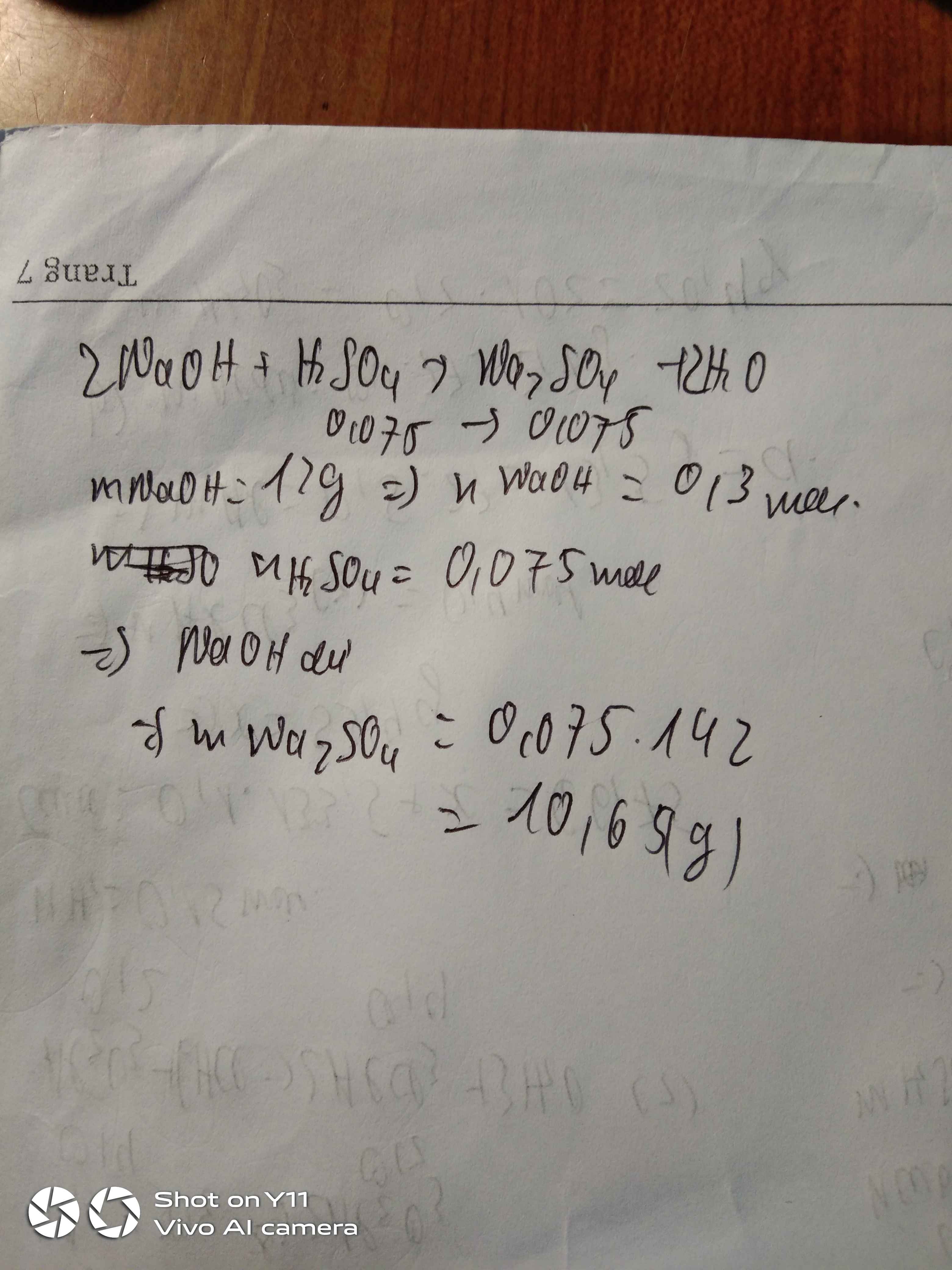\(n_{NaOH}=\dfrac{6\%.200}{40}=0,3\left(mol\right)\\ n_{H_2SO_4}=0,25.0,3=0,075\left(mol\right)\)
PTHH: 2NaOH + H2SO4 ---> Na2SO4 + 2H2O
LTL: \(\dfrac{0,3}{2}>0,075\) => NaOH dư
\(n_{Na_2SO_4}=0,075\left(mol\right)\\ m_{Na_2SO_4}=0,075.142=10,65\left(g\right)\)
\(n_{NaOH}=\dfrac{200.6\%}{40}=0,3\left(mol\right)\\ n_{H_2SO_4}=0,3.0,25=0,075\left(mol\right)\\ pthh:2NaOH+H_2SO_4\rightarrow Na_2SO_4+2H_2O\)
\(LTL:\dfrac{0,3}{2}>\dfrac{0,075}{1}\)
=> NaOH dư , H2SO4 hết
theo pthh : \(n_{Na_2SO_4}=\dfrac{1}{2}n_{H_2SO_4}=0,0375\left(mol\right)\)
=> \(m_{Na_2SO_4}=0,0375.142=5,325\left(g\right)\)