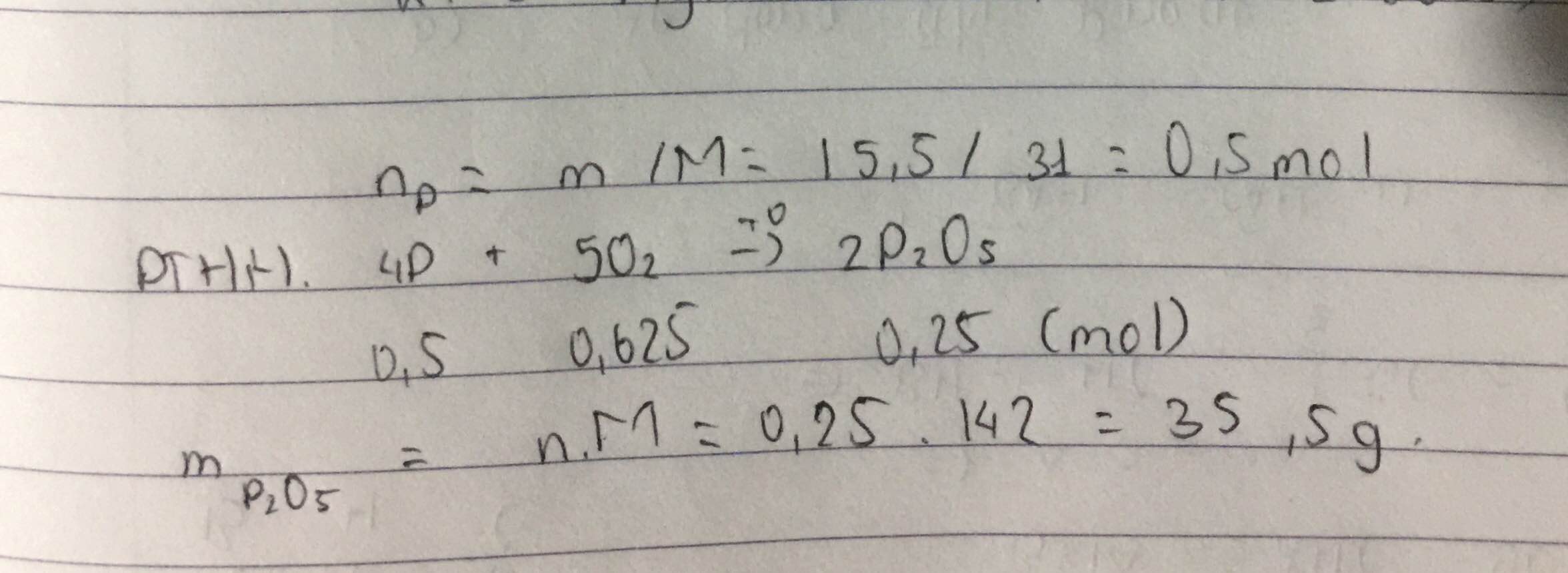nP = 15,5 : 31= 0,5 (mol )
nO2 = (56 : 22,4 ) . 21% = 0,525 (mol )
pthh : 4P+5O2-t--> 2P2O5
LTL
0,5 / 4 > 0,525/5 => P dư
theo pthh , nP2O5 = nO2 = 0,525 (mol)
=> mP2O5 = 0,525 . 142 = 74,55 (g)
\(n_P=\dfrac{m}{M}=\dfrac{15,5}{31}=0,5\left(mol\right)\)
\(n_{O_2}=\dfrac{V}{22,4}=\dfrac{56\times20}{22,4\times100}=0,05\left(mol\right)\)
\(PTHH:4P+5O_2\rightarrow2P_2O_5\)
Ta có: \(\dfrac{0,5}{4}=0,125>\dfrac{0,05}{5}=0,01\)
→ Sau pư O2 hết, P dư
→ Theo \(n_{O_2}\)
Theo pthh: \(n_{P_2O_5}=\dfrac{2}{5}n_{O_2}=\dfrac{2}{5}.0,05=0,02\left(mol\right)\)
\(m_{P_2O_5}=n.M=0,02\times142\times80\%=1,36\left(g\right)\)
\(n_{P\left(phảnứng\right)}=\dfrac{4}{5}n_{O_2}=\dfrac{4}{5}.0,05=0,04\left(mol\right)\)
\(m_{P\left(phảnứng\right)}=n.M=0,04.31=1,24\left(g\right)\)
\(m_{P\left(dư\right)}=15,5-1,24=14,26\left(g\right)\)