\(a) C_2H_5OH +O_2 \xrightarrow{men\ giấm} CH_3COOH + H_2O\\ b) m_{C_2H_5OH} = \dfrac{4,6.8}{100.0,8} = 0,46(gam)\\ n_{CH_3COOH} =n_{C_2H_5OH\ pư} = \dfrac{0,46}{46}.90\% = 0,009(mol)\\ m_{CH_3COOH} = 0,009.60 = 0,54(gam)\\ c) m_{dd\ giấm} = \dfrac{0,54}{5\%} = 10,8(gam)\\ \Rightarrow V_{dd\ giấm} = \dfrac{m}{D} = \dfrac{10,8}{1} = 10,8(ml)\)
Đúng 1
Bình luận (1)








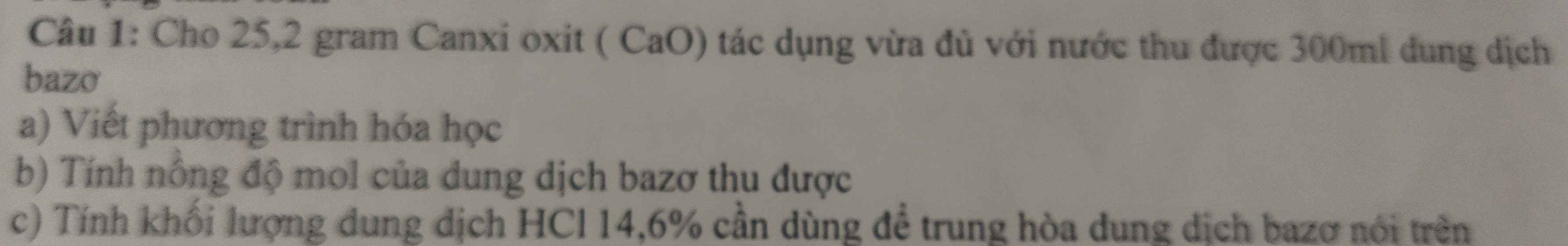






 giúp mình 2 câu này với
giúp mình 2 câu này với